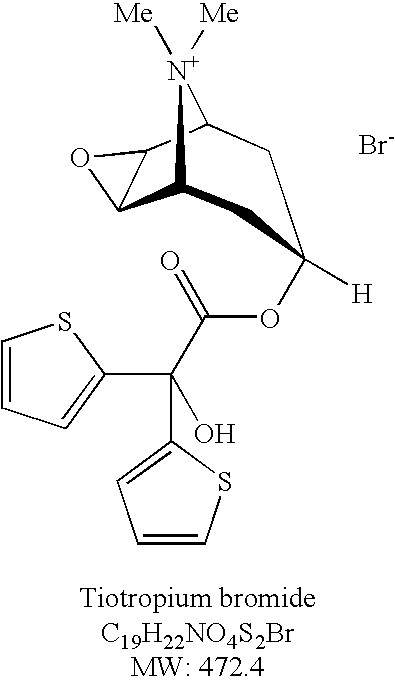
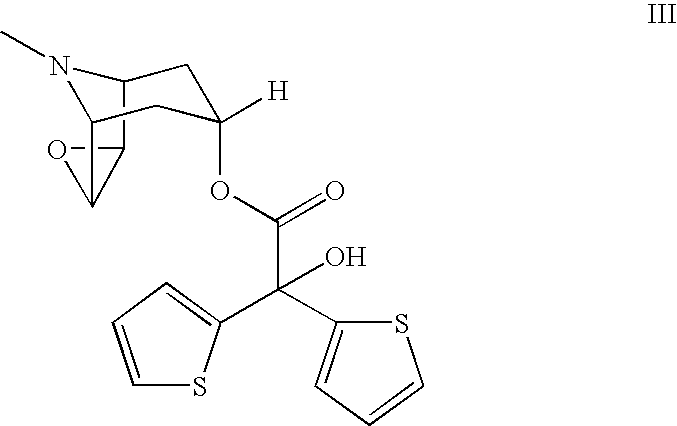
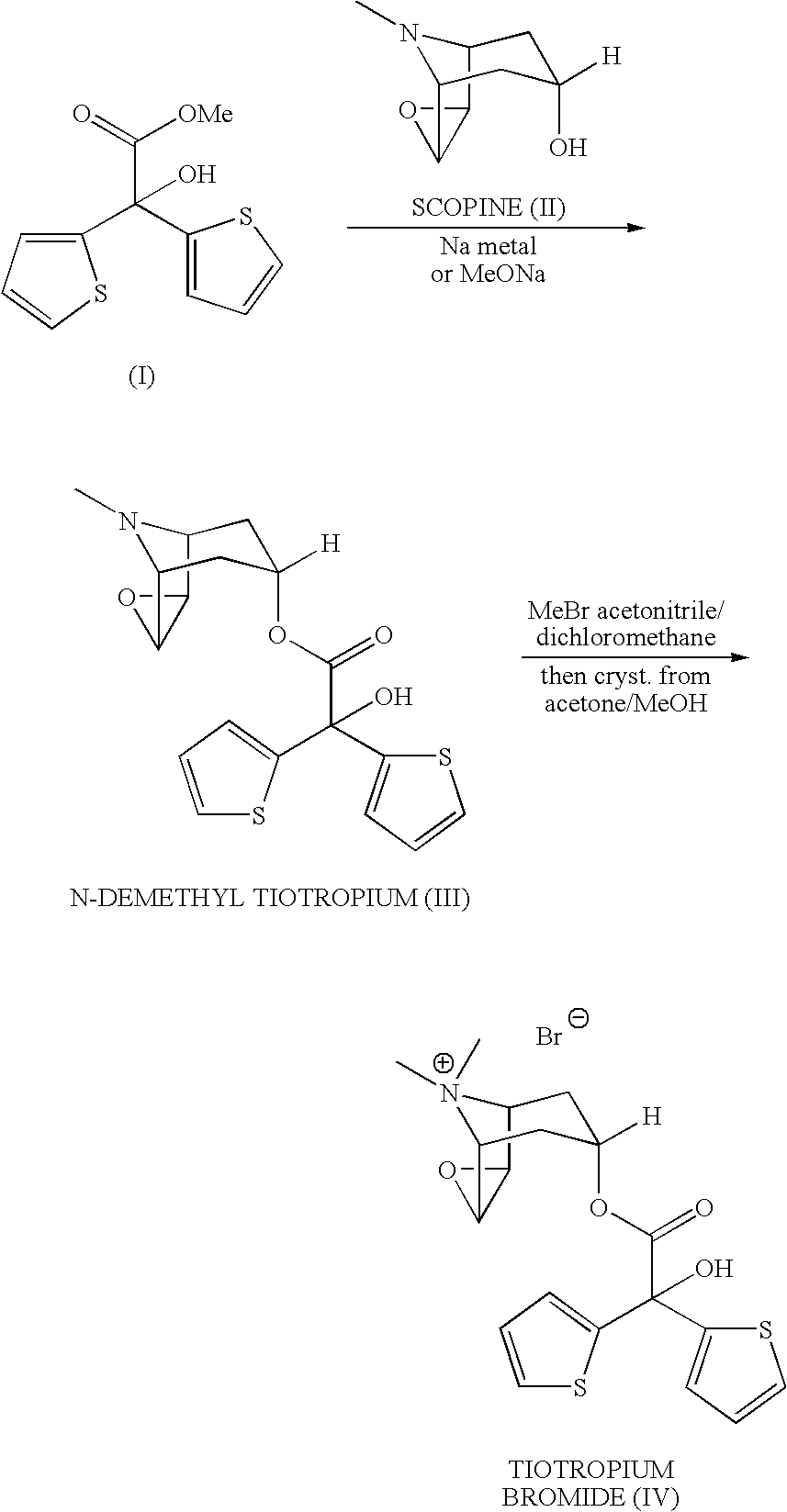
Process for the preparation of tiotropium bromide
a technology of tiotropium bromide and process, applied in the field of improvement, can solve the problems of more complex synthesis methods and unsuitable methods for industrial scale preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Comparative Example: Preparation of Scopine Hydrochloride According to GB '781
[0063] 10.0 g (22.84 mmol) of scopolamine hydrobromide trihydrate was suspended in 100 mL of absolute ethanol, and cooled to about 0° C. Sodium borohydride (4.0 g, 105.7 mmol) was then added portion-wise while maintaining the temperature at a maximum of 30° C. 4.8 mL of water was then added to the reaction mixture. After 3.5 hours, the reaction was completed and 50 mL of diethyl ether was then added. The reaction was then cooled to 0° C., and acidified with 2M hydrochloric acid in diethyl ether to a pH of about 2. The suspension was stirred at room temperature for 30 minutes and then filtered on GochP3. The white solid was dried at 45° C. under vacuum for 4 hours, yielding 9 g of product containing 79% of salts determined by sulphuric ashes.
example 2
Preparation of Methyl di-(2-thienyl)glycolate
[0064] 1050 mL of tetrahydrofuran was loaded in a 2 L round bottomed flask. 22.6 g (0.93 mol) of magnesium turnings were then added, and the mixture was kept at 35° C., while catalytic bromoethane (200 mg, 1.84 mmol) was loaded. 150 g (0.92 mol) of 2-bromothiophene was added dropwise, and after about 15% (13 ml) of reagent was exothermicity was observed. The temperature was maintained at a maximum of 50-55° C., and the remaining 2-bromothiophene was then added. At the end of the addition, the reaction mixture was heated to 65° C. for 1.5 hours to 2 hours, and then cooled to 25° C.
[0065] The Grignard solution thus formed was added drop-wise, in about 2.5 hours to 3 hours, into a solution of dimethyl oxalate (54.3 g, 0.46 mol) dissolved in 300 mL of tetrahydrofuran, while maintaining the temperature at maximum 5-10° C. via cooling bath.
[0066] The solution was kept under stirring for 0.5 hours to 1.0 hours at 5-10° C., and then saturated ...
example 3
Crystallization of Methyl di-(2-thienyl)glycolate in Ethanol 96% / n-heptane
[0072] Crude methyl di-(2-thienyl)glycolate (2.0 g) was dissolved in ethanol 96% (8.0 ml) at 45° C. 16.0 mL of n-heptane were then added drop-wise at 45° C. in 20 minutes. The solution was maintained at 45° C. for hour, and then it was cooled to 0° C. in 1 hour, and left at this temperature for another hour. The solid was filtered on a sintered glass funnel and it was washed once with n-heptane (2 mL). Drying for 6 hours at 50° C. under vacuum yielded 1.4 g of methyl di-(2-thienyl)glycolate (70%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com