SNAP-tag protein tag fluorescence probe with quick specific marking ability
A fluorescent probe and labeling technology, applied in the field of fluorescence imaging, can solve the problems of fast reaction speed, slow reaction speed, uncontrollable quantity and position, etc., and achieve the effect of easy purification and low-cost synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
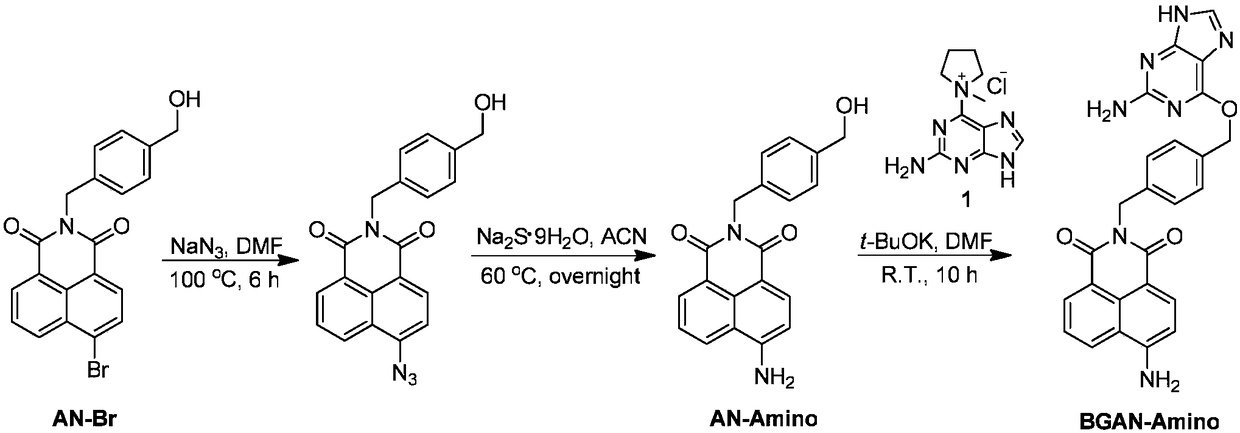
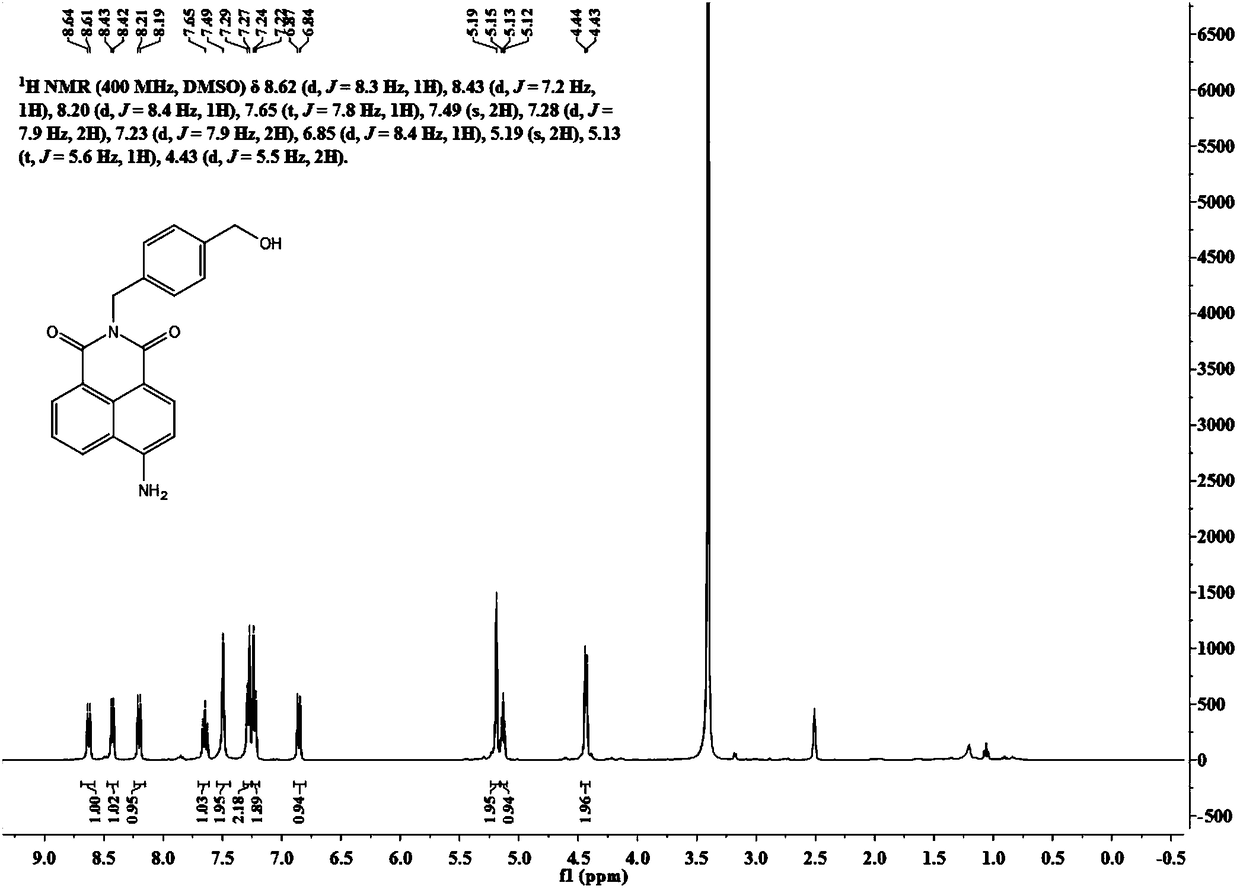
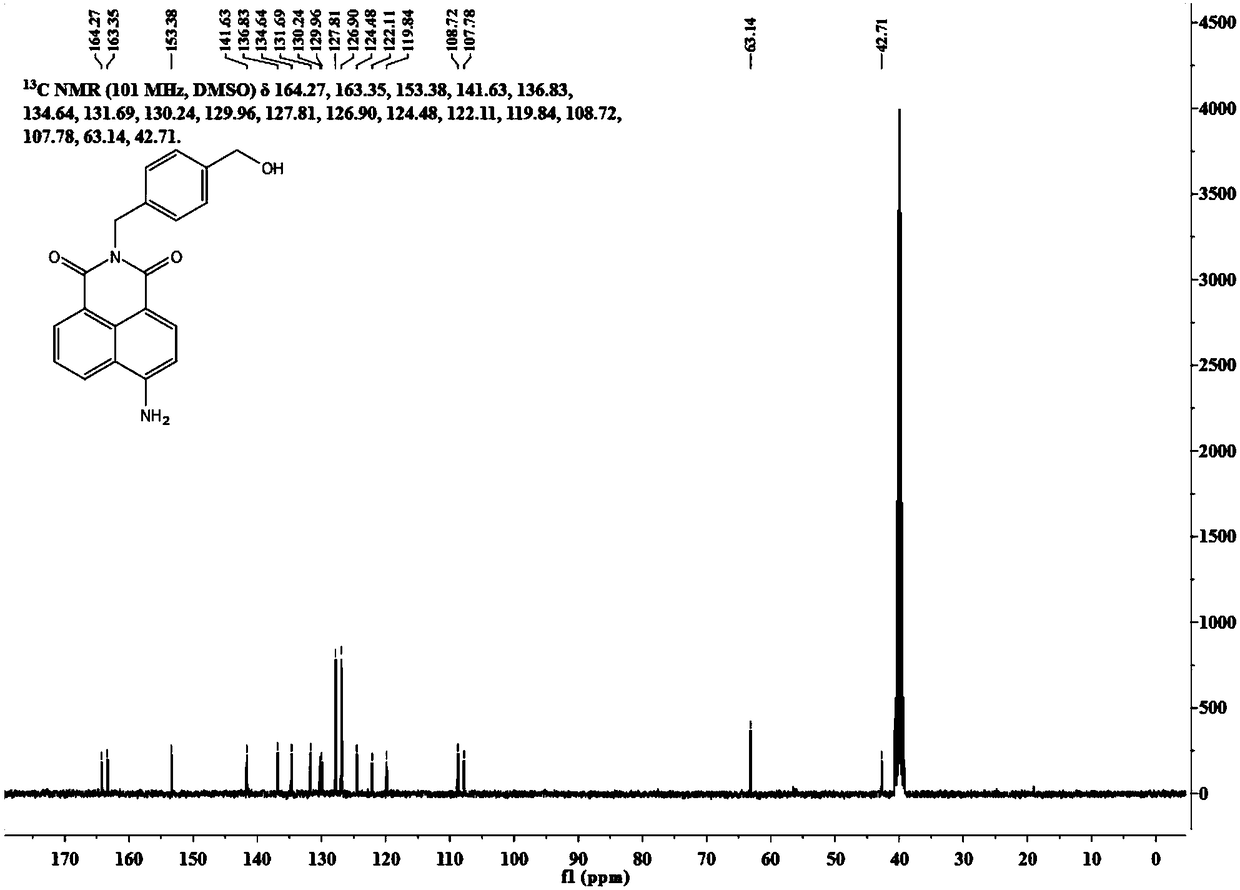
[0029] A method for synthesizing a SNAP-tag protein-labeled fluorescent probe with specific and rapid labeling ability.
[0030] (1) Synthesis of intermediate 4-amino-N-(4-hydroxymethylbenzyl) 1,8-naphthalimide:
[0031] Put 4-bromo-N-(4-hydroxymethylbenzyl)1,8-naphthalimide (200mg, 0.50mmol) in a 100mL one-necked bottle, and add 10mL of N,N-dimethylformamide. Sodium azide (100 mg, 1.50 mmol) was dissolved in 1 mL of water and added to the mixture, and the reaction solution was heated to 90°C. After 6h, the heating was stopped, and the reaction solution was poured into ice water to settle and suction filtered to obtain a dark yellow solid. The resulting solid was dissolved in 50 mL of acetonitrile, and sodium sulfide nonahydrate (720 mg, 3.00 mmol) was added. The reaction solution was heated to 60°C for 12h. Silica gel column separation (200-300 mesh) and dichloromethane and methanol (200:1-100:1) were used as developing solvents to obtain 45 mg of a yellow solid with a yie...
Embodiment 2
[0040] A method for synthesizing a SNAP-tag protein-labeled fluorescent probe with specific and rapid labeling ability.
[0041] (1) Synthesis of intermediate 4-amino-N-(4-hydroxymethylbenzyl) 1,8-naphthalimide:
[0042] Put 4-bromo-N-(4-hydroxymethylbenzyl)1,8-naphthalimide (500mg, 1.25mmol) in a 100mL one-necked bottle, and add 50mL of N,N-dimethylformamide. Sodium azide (500 mg, 7.50 mmol) was dissolved in 2 mL of water and added to the mixture, and the reaction solution was heated to 100°C. After 8 hours, the heating was stopped, and the reaction solution was poured into ice water to settle and suction filtered to obtain a dark yellow solid. The resulting solid was dissolved in 80 mL of acetonitrile, and sodium sulfide nonahydrate (2500 mg, 10.4 mmol) was added. The reaction solution was heated to 70°C for 15h. Silica gel column separation (200-300 mesh) and dichloromethane and methanol (200:1-100:1) were used as developing solvents to obtain 200 mg of a yellow solid wi...
Embodiment 3
[0046] A method for synthesizing a SNAP-tag protein-labeled fluorescent probe with specific and rapid labeling ability.
[0047] (1) Synthesis of intermediate 4-amino-N-(4-hydroxymethylbenzyl) 1,8-naphthalimide:
[0048] Put 4-bromo-N-(4-hydroxymethylbenzyl)1,8-naphthalimide (1000mg, 2.50mmol) in a 100mL one-necked bottle, and add 75mL of N,N-dimethylformamide. Sodium azide (500 mg, 7.50 mmol) was dissolved in 2 mL of water and added to the mixture, and the reaction solution was heated to 110°C. After 10 h, the heating was stopped, and the reaction solution was poured into ice water to settle and suction filtered to obtain a dark yellow solid. The resulting solid was dissolved in 80 mL of acetonitrile, and sodium sulfide nonahydrate (3600 mg, 15.00 mmol) was added. The reaction solution was heated to 80°C for 18h. Silica gel column separation (200-300 mesh) and dichloromethane and methanol (200:1-100:1) were used as developing solvents to obtain 300 mg of a yellow solid wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com