Full-spectrum optical switch molecule as well as synthesis and application thereof
An optical switching, full-spectrum technology, applied in the field of fluorescence imaging, which can solve the problems of reduced positioning accuracy, poor anti-bleaching performance of fluorescent proteins, and limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
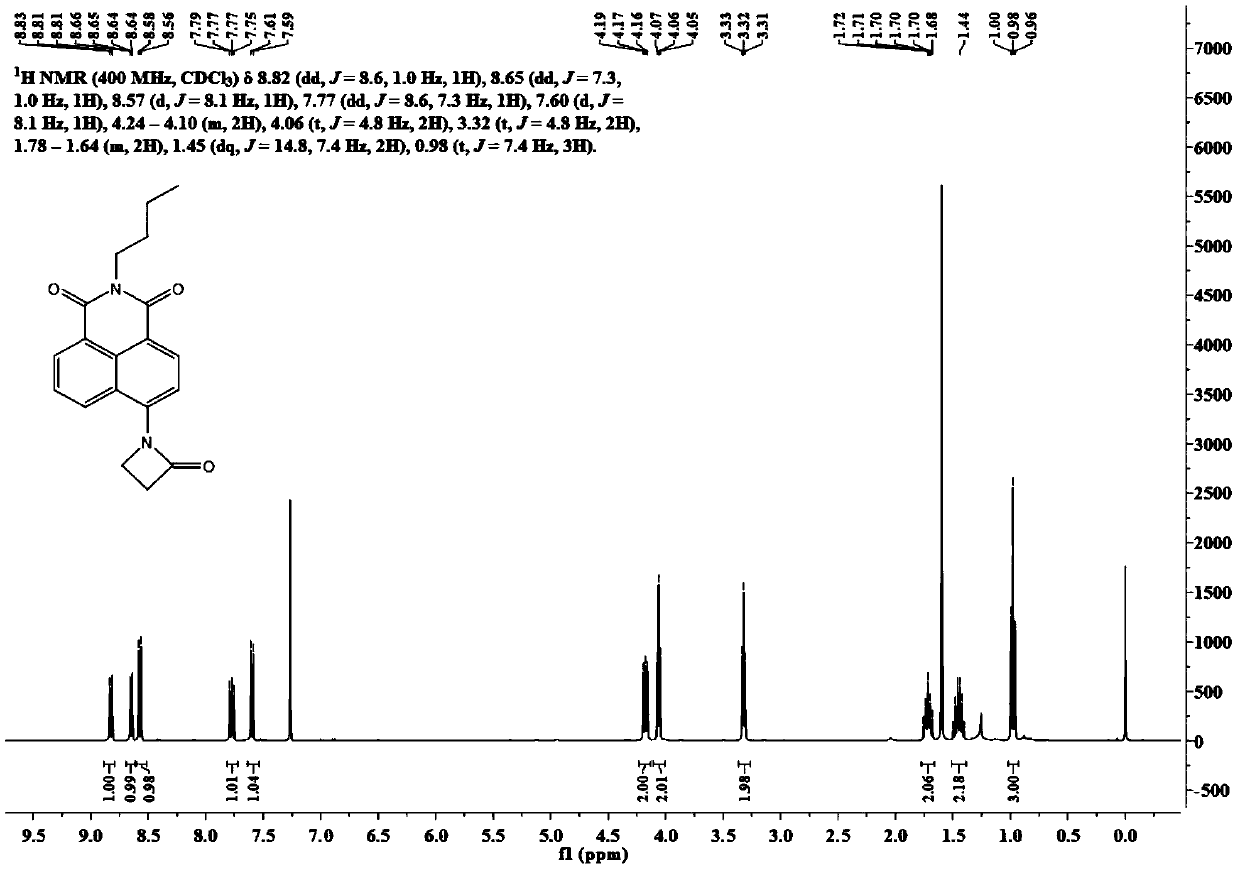
[0088] Synthesis of AB-405
[0089] Synthesis of intermediate N-butyl-4-(3-chloro)propionamido-1,8-naphthoimide (ClPAm):
[0090]
[0091] N-butyl-4-amino-1,8-naphthalimide (200 mg, 0.75 mmol) was dissolved in 100 mL of tetrahydrofuran, and 1.25 mL of 3-chloropropionyl chloride was added dropwise to the reaction solution at 0°C. After the dropwise addition, the mixture was transferred to room temperature for 6 h. After removing the solvent under reduced pressure, wash the residue with 63mL of water, filter with suction to obtain a white filter cake, wash the filter cake with 25mL of methanol, and dry in vacuo to obtain N-butyl-4-(3-chloro)propionamido-1,8-naphthalene Imide 180 mg, yield 67%. Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0092] 1 H NMR (400MHz, CD 3 CN)δ8.91(s,1H),8.59(dd,J=7.3,0.9Hz,1H),8.54(d,J=8.1Hz,1H),8.52–8.48(m,1H),8.29(d, J=8.1Hz, 1H), 7.85(dd, J=8.5, 7.3Hz, 1H), 4.15–4.10(m, 2H), 3.98(t, J=6.3Hz, 2H), 3.08(t, J=6.3 Hz...
Embodiment 2
[0099] Synthesis of AB-405
[0100] Synthesis of intermediate N-butyl-4-(3-chloro)propionamido-1,8-naphthoimide (ClPAm):
[0101]
[0102] N-butyl-4-amino-1,8-naphthalimide (200 mg, 0.75 mmol) was dissolved in 8 mL of tetrahydrofuran, and 0.5 mL of 3-chloropropionyl chloride was added dropwise to the reaction solution at 0°C. After the dropwise addition, the mixture was transferred to room temperature for 10 h. After removing the solvent under reduced pressure, wash the residue with 25 mL of water, filter with suction to obtain a white filter cake, wash the filter cake with 10 mL of methanol, and dry in vacuo to obtain N-butyl-4-(3-chloro)propionamido-1,8-naphthalene Imide 142 mg, yield 53%.
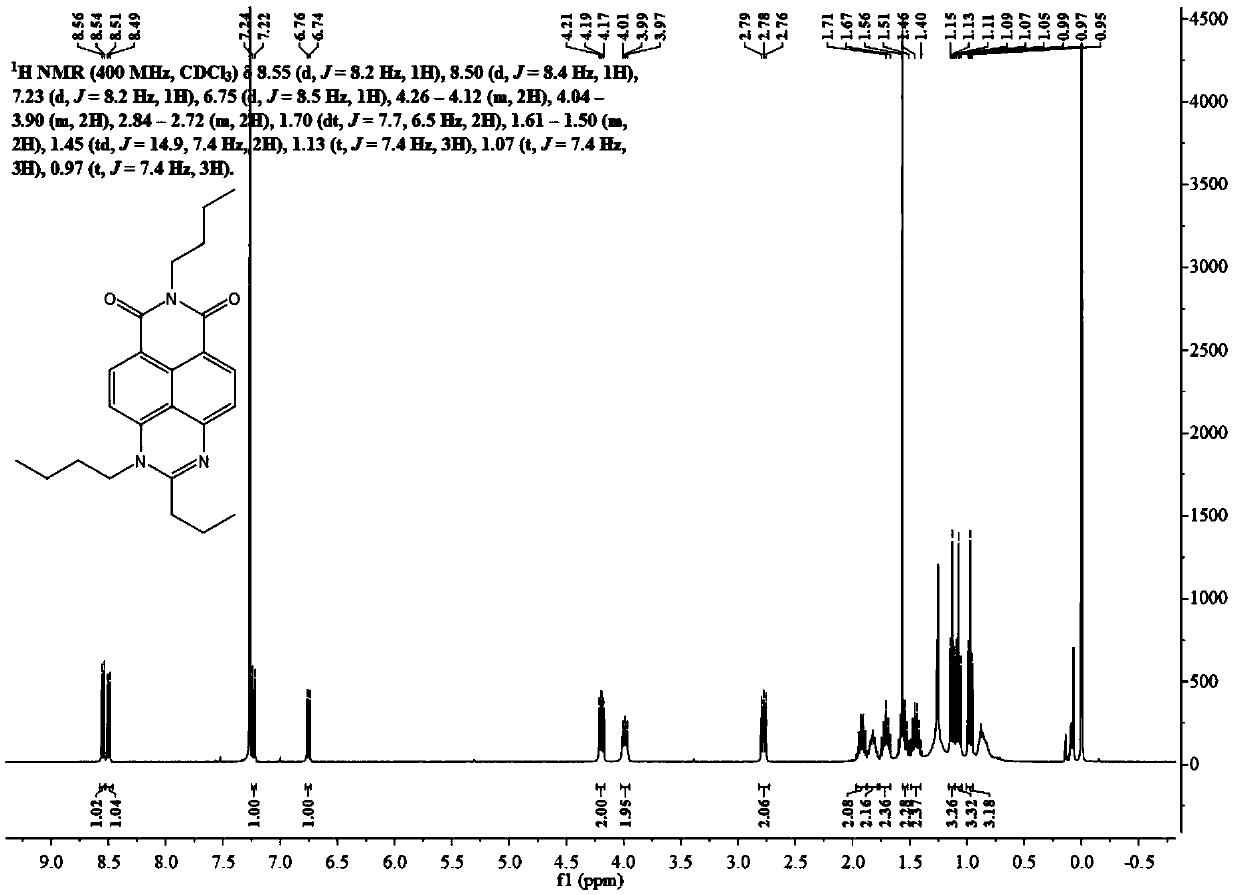
[0103] Synthesis of Dye N-Butyl-4-Cyclobutanylamido-1,8 Naphthalimide (PAm):
[0104]
[0105] N-butyl-4-(3-chloro)propionamido-1,8-naphthalimide (100 mg, 0.28 mmol) was dissolved in 20 mL of acetonitrile, and 400 mg of potassium carbonate was added thereto. The temperature of ...
Embodiment 3
[0108] Synthesis of AB-405
[0109] Synthesis of intermediate N-butyl-4-(3-chloro)propionamido-1,8-naphthoimide (ClPAm):
[0110]
[0111] N-butyl-4-amino-1,8-naphthalimide (200 mg, 0.75 mmol) was dissolved in 100 mL of tetrahydrofuran, and 5 mL of 3-chloropropionyl chloride was added dropwise to the reaction solution at 0°C. After the dropwise addition, the mixture was transferred to room temperature for 8 h. After removing the solvent under reduced pressure, wash the residue with 25 mL of water, filter with suction to obtain a white filter cake, wash the filter cake with 60 mL of methanol, and dry in vacuo to obtain N-butyl-4-(3-chloro)propionamido-1,8-naphthalene Imide 161 mg, yield 60%.
[0112] Synthesis of Dye N-Butyl-4-Cyclobutanylamido-1,8 Naphthalimide (PAm):
[0113]
[0114] N-butyl-4-(3-chloro)propionamido-1,8-naphthalimide (100 mg, 0.28 mmol) was dissolved in 15 mL of acetonitrile, and 200 mg of potassium carbonate was added thereto. The reaction solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com