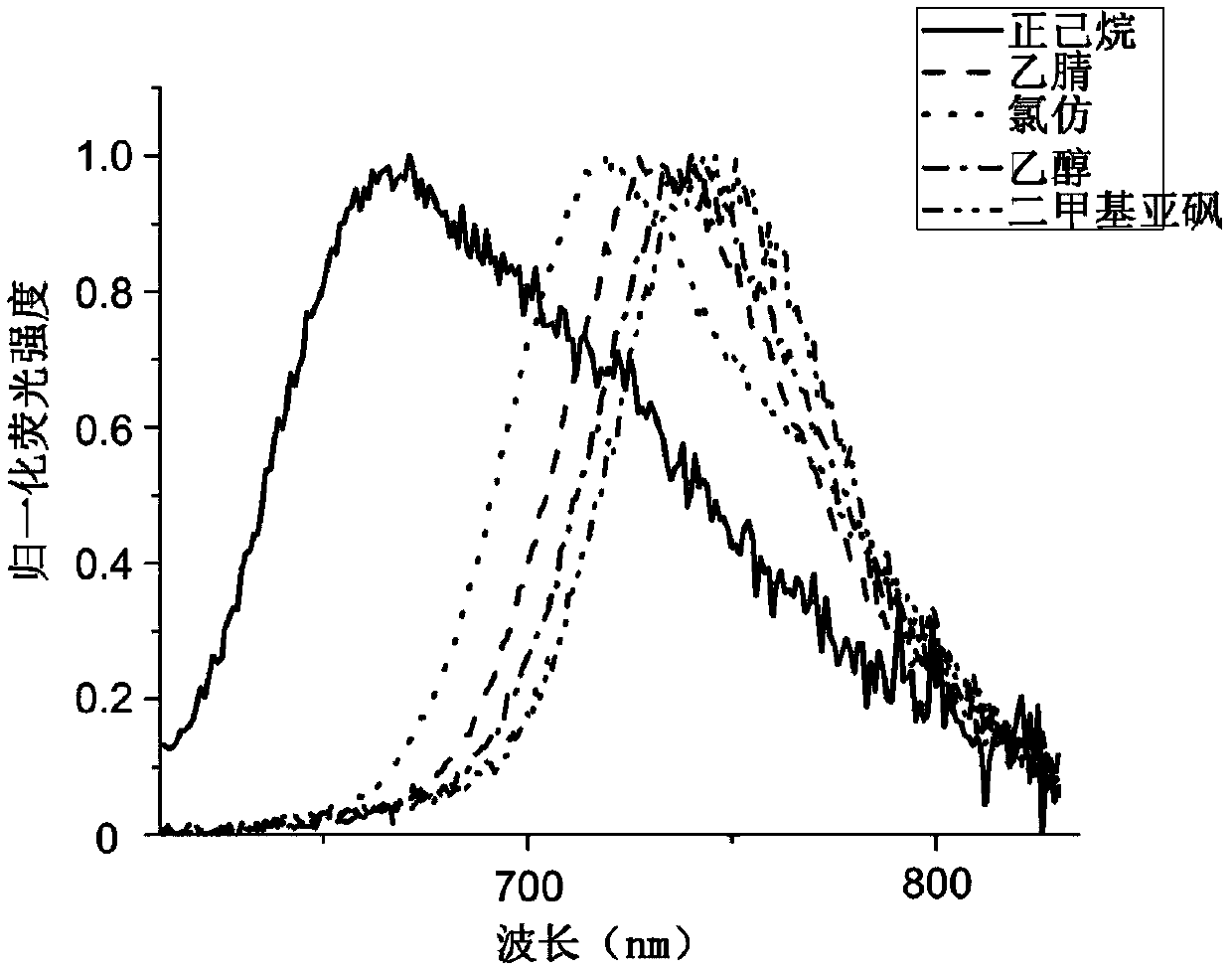
Fluorescent dye for lipid droplet labeling, and synthesis method and application thereof
A technology of fluorescent dye and synthesis method, which is applied in the field of lipid droplet-labeled fluorescent dye and its synthesis, can solve the problems that are not conducive to in vivo fluorescence imaging, and achieve the effects of photostability and brightness improvement, simple purification, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis method of lipid droplet fluorescent dye MLD-DAzi.
[0041] Synthesis of intermediate N-methyl-9,10-dibromo-1,6,7,12-tetrachloroperylimide (MLD-DBr):
[0042]
[0043] Dissolve 1,6,7,12-tetrachloro-9,10-dibromo-3,4-perylene anhydride (1.2 g, 1.96 mmol) in 50 mL of a mixture of acetic acid and N-methylpyrrolidone (1:1, V / V), and then dropwise added methylamine 120mg therein. After reacting at 100°C for 3 h, the reaction solution was poured into 200 mL of ice water, settled and filtered to obtain a black solid. The black solid was separated through a silica gel column (petroleum ether:dichloromethane=1:1, V / V) to obtain 482 mg of a red solid with a yield of 39%. Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0044] 1 H NMR (400MHz, CDCl 3 )δ8.59(s,2H),8.14(s,2H),4.33(s,3H).
[0045] Synthesis of the dye N-methyl-9,10-di-aziridinyl-1,6,7,12-tetrachloroperylimide:
[0046]
[0047] N-butyl-1,6,7,12-tetrachloro-9,10-dibromo-3,4-pe...
Embodiment 2
[0051] Synthesis method of lipid droplet fluorescent dye BuLD-DAze.
[0052] Synthesis of intermediate N-butyl-9,10-dibromo-1,6,7,12-tetrachloroperyleneimide:
[0053]
[0054] Dissolve 1,6,7,12-tetrachloro-9,10-dibromo-3,4-perylene anhydride (1.2g, 1.96mmol) in 144mL of a mixture of acetic acid and N-methylpyrrolidone (4:1, V / V), and then add n-butylamine (428mg, 5.86mmol) dropwise thereto. After reacting at 140°C for 1 h, the reaction solution was poured into 200 mL of ice water, settled and filtered to obtain a black solid. The black solid was separated through a silica gel column (petroleum ether:dichloromethane=1:1, V / V) to obtain 600 mg of a red solid with a yield of 46%. Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0055] 1 H NMR (400MHz, CDCl 3 )δ8.59(s,2H),8.14(s,2H),4.38–4.11(m,2H),1.94–1.66(m,2H),1.56–1.38(m,2H),0.99(t,J= 7.1Hz, 3H).
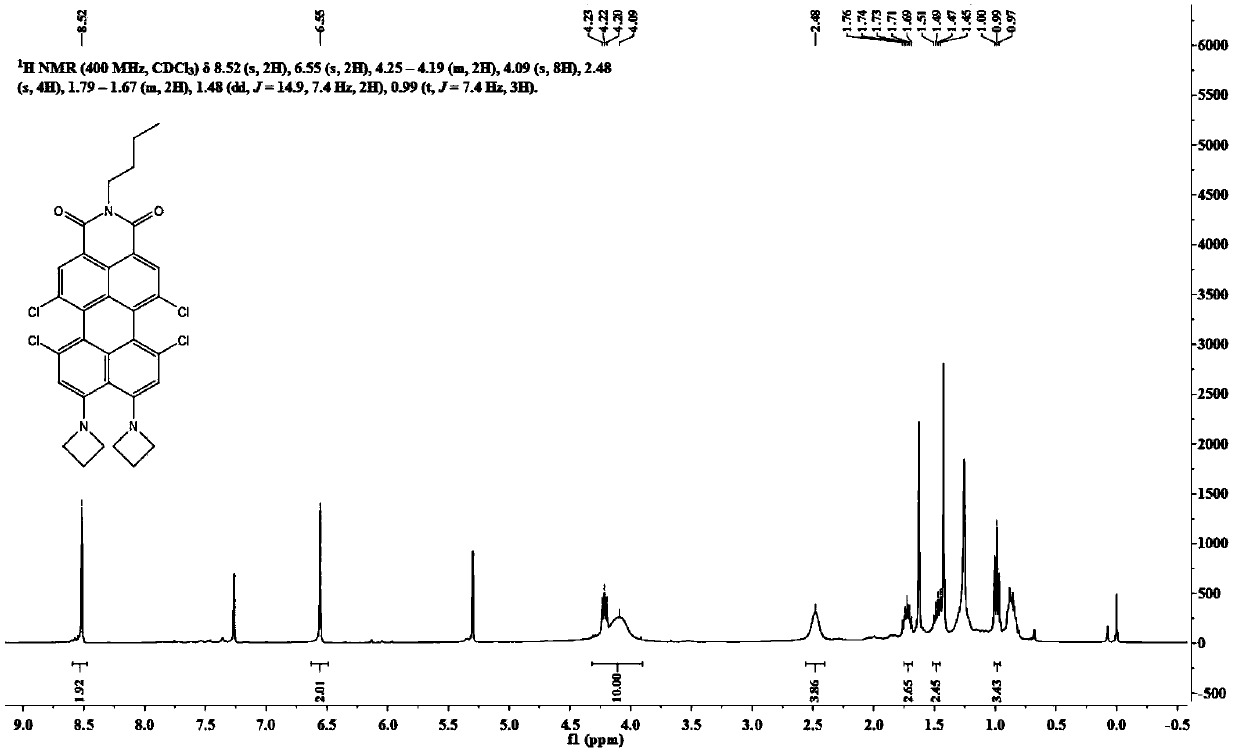
[0056] Synthesis of the dye N-butyl-9,10-di-azetidinyl-1,6,7,12-tetrachloroperylimide:
[0057...
Embodiment 3
[0062] Synthesis method of lipid droplet fluorescent dye OLD-DAze.
[0063] Synthesis of intermediate N-(2-(2-hydroxy)-ethoxy)ethyl-9,10-dibromo-1,6,7,12-tetrachloroperyleneimide:
[0064]
[0065] Dissolve 1,6,7,12-tetrachloro-9,10-dibromo-3,4-perylene anhydride (1.2g, 1.96mmol) in 24mL of a mixture of acetic acid and N-methylpyrrolidone (2:1, V / V), and then added diglycolamine (303mg, 8.79mmol) dropwise thereto. After reacting at 100°C for 6 h, the reaction solution was poured into 150 mL of ice water, settled and filtered to obtain a black solid. The black solid was separated through a silica gel column (petroleum ether:dichloromethane=1:1-1:4, V / V) to obtain 380 mg of a red solid with a yield of 55%. Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
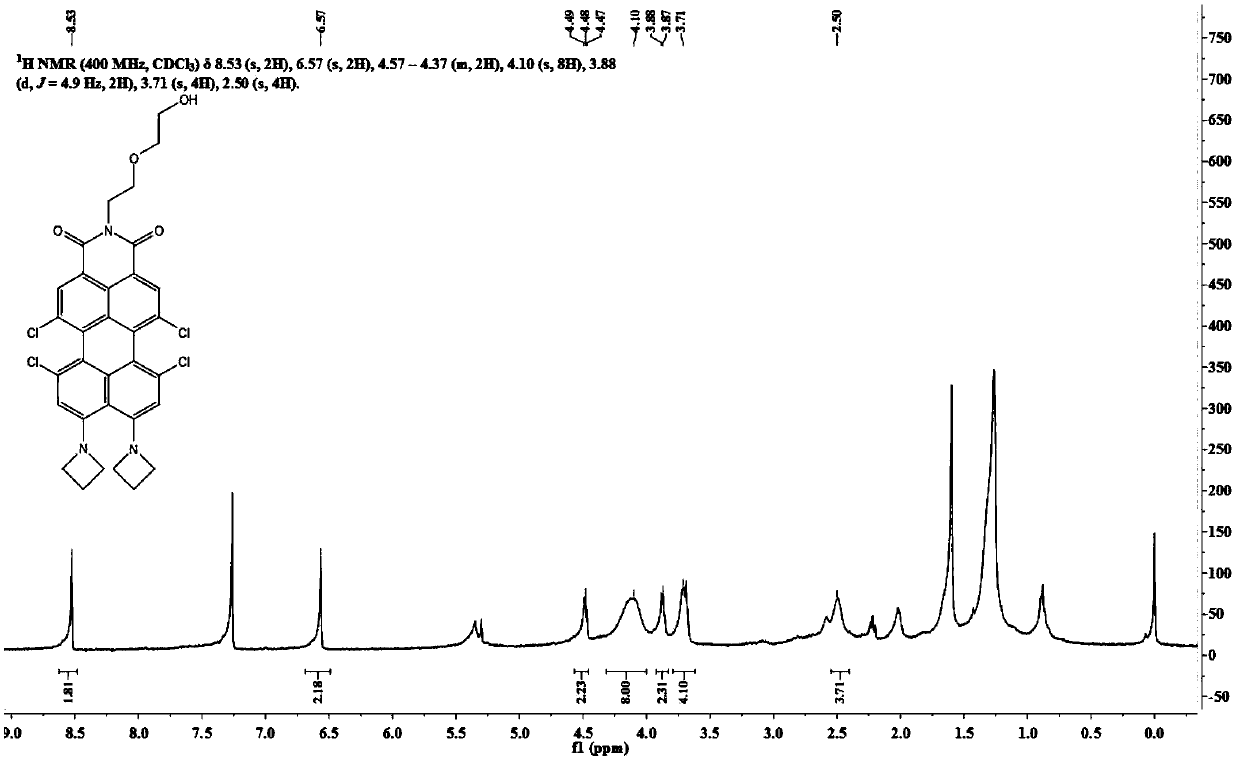
[0066] 1 H NMR (400MHz, CDCl 3 )δ8.60(s,2H),8.13(s,2H),4.67–4.41(m,2H),3.88(d,J=5.3Hz,2H),3.71(d,J=4.5Hz,2H), 3.67(d,J=3.6Hz,2H),2.38(s,1H).
[0067] Dye N-(2-(2-hydroxy)-ethoxy)ethyl-9,10-di-aze...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com