Target protein expression with laetiporus sulphureus mushroom lectin N-acetyllactosamine binding domain as fusion tag and purification method thereof
A technology of acetyllactosamine and fusion protein, which is applied in the field of genetic engineering, can solve the problems of affecting gene expression level, protein insolubility, low expression of tagged protein and target protein, and achieve the effect of reducing purification cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the synthesis of LSL gene and the construction of label gene
[0060] The DNA analysis and RNA structure prediction of the LSLa gene (its nucleotide sequence is shown in SEQ ID NO.3) encoding the N-acetyllactosamine binding domain of the sulfur bacteria mushroom lectin was artificially synthesized without changing the natural amino acid sequence The LSL gene encoding the N-acetyllactosamine binding domain of the sulfur bacteria mushroom lectin, the nucleotide sequence of the artificially synthesized LSL gene is the nucleotide sequence shown in SEQ ID NO.1.
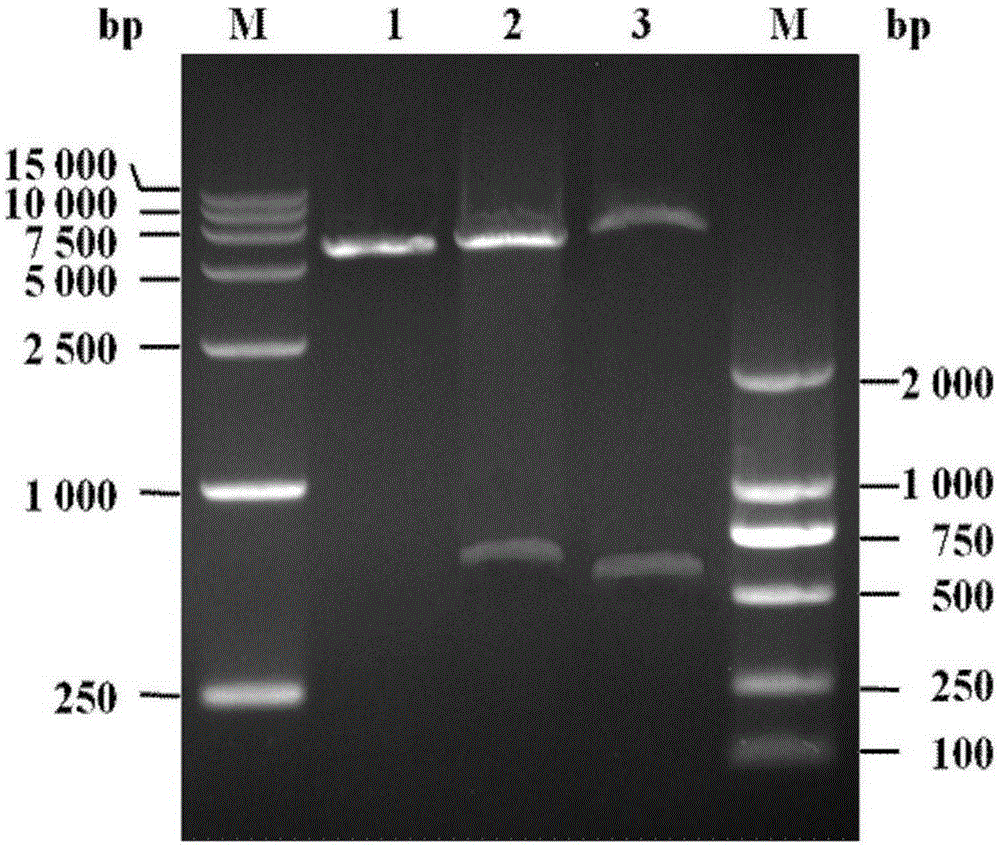
[0061]Add the NcoI restriction endonuclease recognition site at the 5' end of the above-mentioned artificially synthesized LSL gene sequence, and add the KpnI restriction endonuclease recognition site, TEV protease recognition site and The BamHI restriction endonuclease recognizes the site to form a tag gene, and then clones the tag gene into the pET32a(+) plasmid to obtain the pET32a-LSL plasmid conta...
Embodiment 2
[0062] Example 2: Construction of an expression vector containing the LSL gene encoding the sulfur bacteria mushroom lectin N-acetyllactosamine binding domain
[0063] 1. Construction of pET28a-LSL expression vector
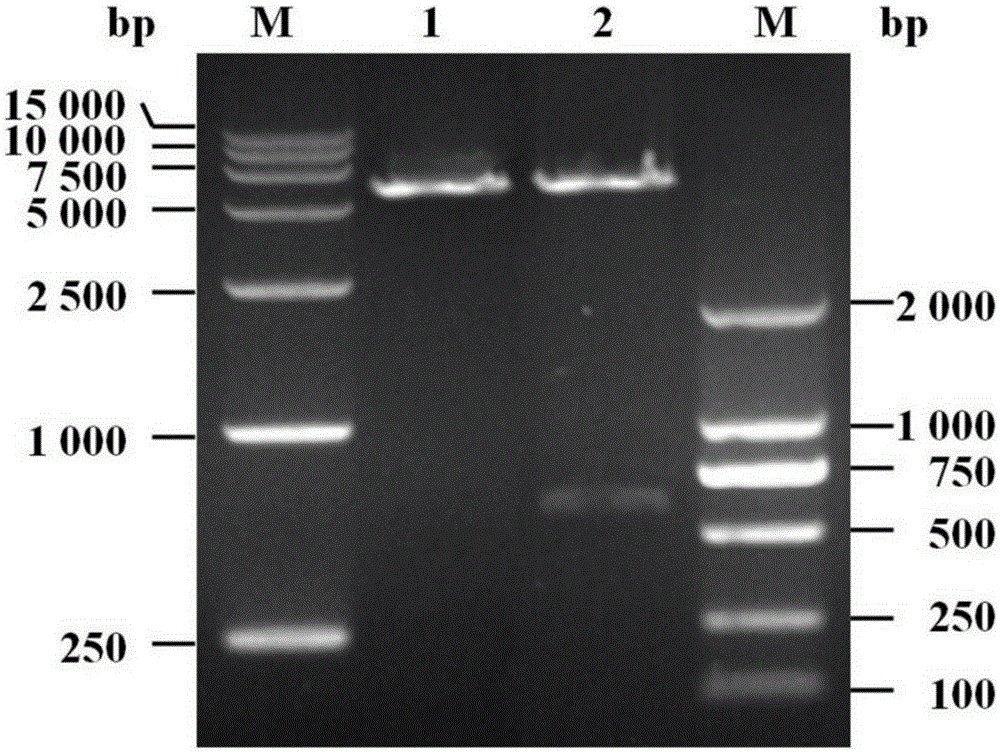
[0064] The pET32a-LSL plasmid containing the tag gene synthesized in Example 1 was double digested with NcoI and BamHI, and the tag gene fragment containing both the LSL gene and the TEV protease recognition site was recovered by agarose gel electrophoresis; the recovery was obtained by T4DNA ligase The tag gene fragment containing both the LSL gene and the TEV protease recognition site was connected to the pET28a(+) expression vector after double digestion with NcoI and BamHI; then the ligation product was transformed into E. coli competent cells DH5α, and The transformed DH5α was spread onto the LB solid culture plate containing kanamycin and cultured overnight at 37°C. The next day, a single colony was picked and placed in 5ml of LB liquid medium containing k...
Embodiment 3
[0074] Embodiment 3: Expression of the fusion protein containing LSL protein tag
[0075] 1. Expression of fusion protein containing LSL protein tag and Cap protein (LSL-Cap fusion protein for short)
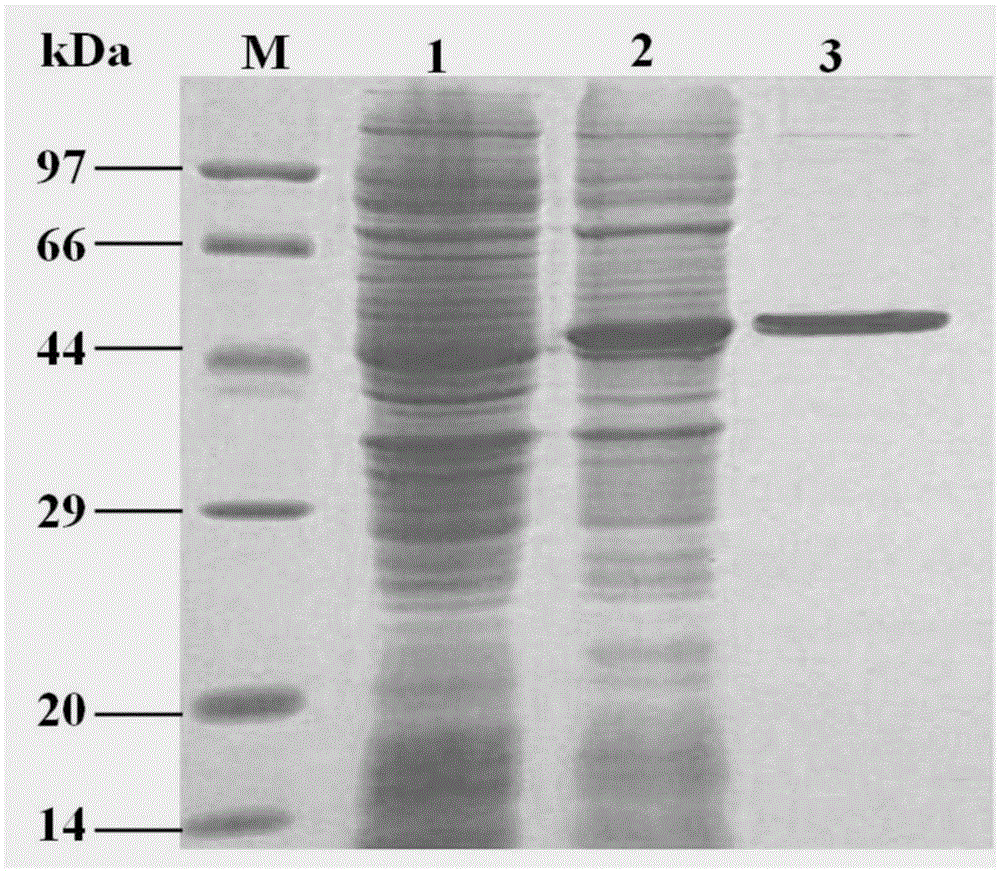
[0076] Expression method of fusion protein: Transform the expression vector pET28a-LSL-Cap into E.coliBL21(DE3) host bacteria, and then apply the transformed E.coliBL21(DE3) to the LB solid culture plate containing kanamycin , overnight at 37°C. The next day, pick a single colony and place it in 5ml LB liquid medium containing kanamycin, and culture it at 200rpm at 37°C for 12h; At 37°C, 200rpm shaking culture until the OD600≈0.8 of the bacterial liquid, adding isopropylthiogalactopyranoside (IPTG) with a final concentration of 1.0mmol / L, and inducing culture at 25°C, 200rpm for 6h. Centrifuge the cultured bacterial solution at 8000rpm for 5 minutes, discard the supernatant, collect the bacterial cell pellet, and add bacterial cell pellet with bacterial cell pellet to resuspen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com