A highly expressed water-soluble heparanase I fusion protein and its coding gene
A fusion protein and coding gene technology, applied in the field of genetic engineering, can solve the problems of low expression, poor water solubility, low activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, expression of heparanase I fusion protein pColdTF-HepI
[0027] 1. Cloning of Flavobacterium heparinase I coding sequence with signal peptide removed
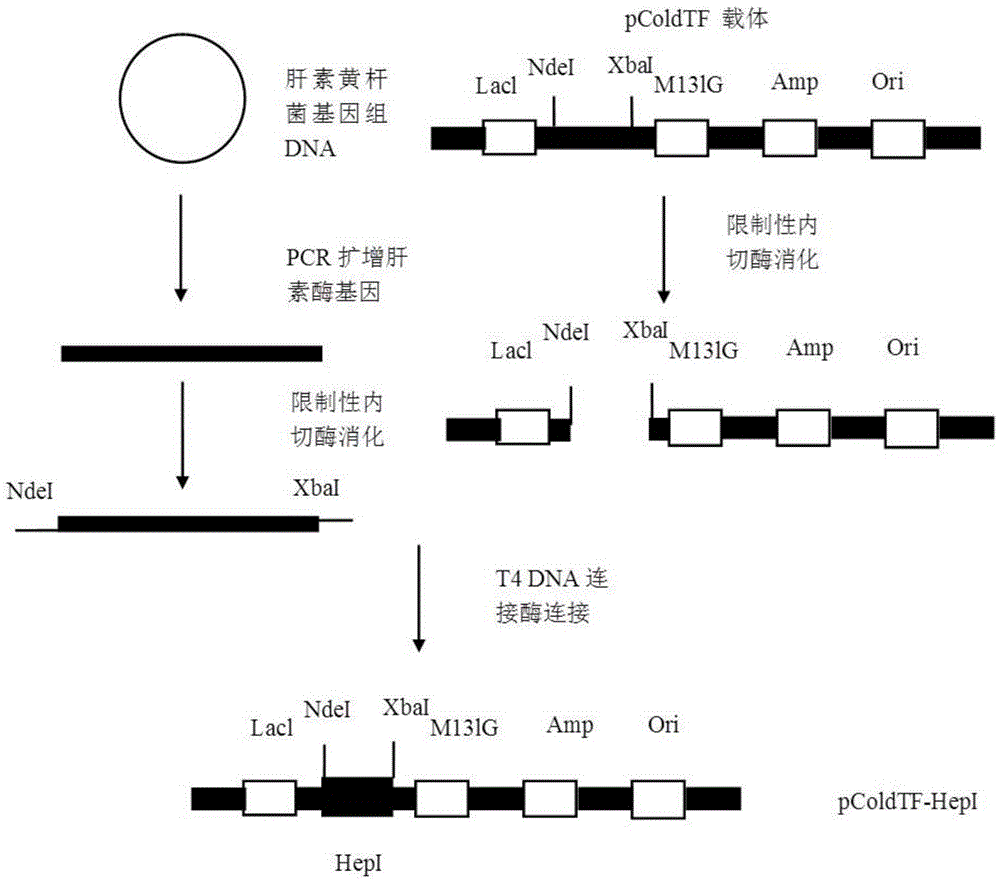
[0028] The construction process of the expression vector pColdTF-HepI is as follows: figure 1 As shown, the specific process is as follows:
[0029] 1. Design and synthesis of primers
[0030] The DNA sequence of heparinase I from Flavobacterium heparinum (Su, H., Blain, F., Musil, R.A., Zimmermann, J.J., Gu, K. and Bennett, D.C. Isolation and expression in Escherichia aliofhep Bandhep C, genescoding for theglycosaminoglycan-degrading enzyme II, genecoding for theglycosaminoglycan-degrading enzyme II, genecoding for theglycosaminoglycan-degrading enzyme II, heparinase I was obtained through Genbank query , fromFlavobacterium heparinum.Appl.Environ.Microbiol.1996, 62, 2723-2734), and then design primers according to the DNA sequence of Flavobacterium heparinase I that encodes the base of the signal peptide...
Embodiment 2
[0056] Example 2, Purification of heparanase I fusion protein pColdTF-HepI by nickel column
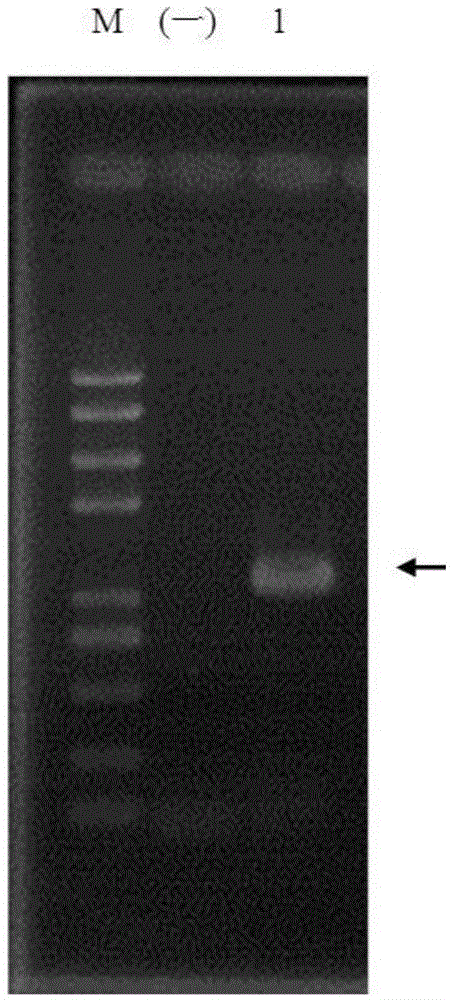
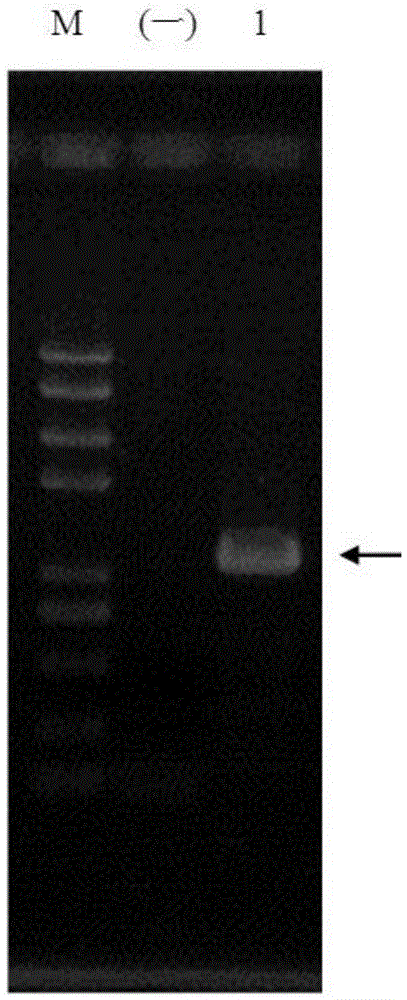
[0057] pColdTF-HepI was transformed into Escherichia coli strain BL21(DE3) (purchased from Novagen, USA), and then induced expression with recombinase according to the operation steps provided by the company. And the HepI fusion protein was purified with NiSepharose6FastFlow (GE) gel, and the purification conditions were operated according to the product manual of GE Company. The purification of recombinant pColdTF-HepI was detected by polyacrylamide gel electrophoresis, and the results were as follows: Figure 5 As shown in the No. 5 swimming lane of , the purified recombinant HepI fusion protein presents a single band on the electrophoresis gel, and the position coincides with the predicted molecular weight.
Embodiment 3
[0058] Embodiment 3, the enzyme activity assay of HepI fusion protein
[0059] The mass concentration was 1% heparin, pColdTF-HepI enzyme solution, 5 times buffer solution (250mM Tris-HCl, 500mMNaCl, 10mM CaCl 2 , pH7.9) and water were mixed according to the ratio of 2:1:2:5 (volume ratio), reacted at optimum temperature and optimum pH for 2-10min, and measured enzyme activity according to the aforementioned ultraviolet method (Yamagata, Saito et al. .1968), while measuring the protein content of the HepI fusion protease liquid with the protein quantitative kit purchased from Kangwei Century Company, the results showed that the specific activity of the recombinant HepI fusion protein to heparin was 200U / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com