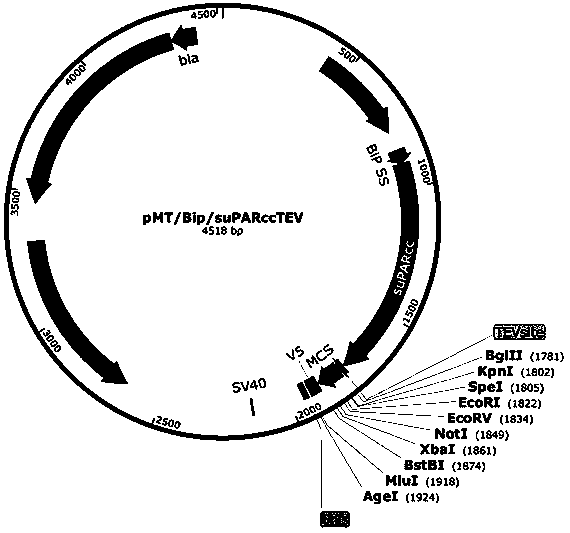
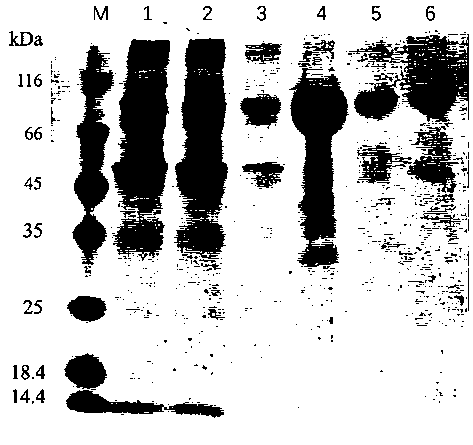
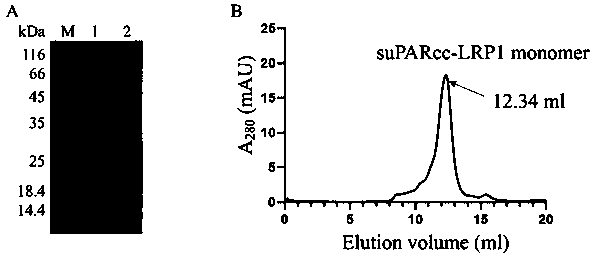
Application of urokinase receptor stable mutant suPARcc in eukaryotic extracellular protein expression
A technology for urokinase receptors and extracellular proteins, applied in the direction of receptors/cell surface antigens/cell surface determinants, animal/human proteins, fusion with protease sites, etc., can solve target protein mutual interference, inappropriate target protein High-efficiency expression and purification issues, to achieve the effects of efficient purification, specific binding, and reduced purification costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The Drosophila Schneider 2 (S2 for short) insect cell expression system is an expression system with post-translational modifications that are very close to mammalian cells. Compared with mammalian expression systems, it does not require CO 2 , can be cultured in suspension, and the number of cells can reach 3×10 at the same time 7 / ml, much higher than the cell number of mammalian cell 293F suspension culture (3×10 6 / ml), which has the advantages of high expression yield and easy amplification, and is especially suitable for expressing proteins that require post-translational modification to be active.
[0023] The construction process of the novel fusion tag protein of the present invention and the effect of the expression system of the novel fusion protein will be described in detail below through examples in conjunction with the accompanying drawings, but the scope of the present invention is not limited in any way.
[0024] In the examples, if the specific experi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com