Genetic engineering bacterium expressing snake venom kininogenase as well as constructing method and application thereof
A technology of genetically engineered bacteria and kininogenase, which is applied in the field of genetically engineered bacteria that efficiently express human snake venom kininogenase, can solve the problems that drugs cannot maximize their effects, cannot be administered intravenously, and have low purity , to achieve the effect of simplifying the follow-up purification work, low cost and high purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Amplification of snake venom kininogenase gene
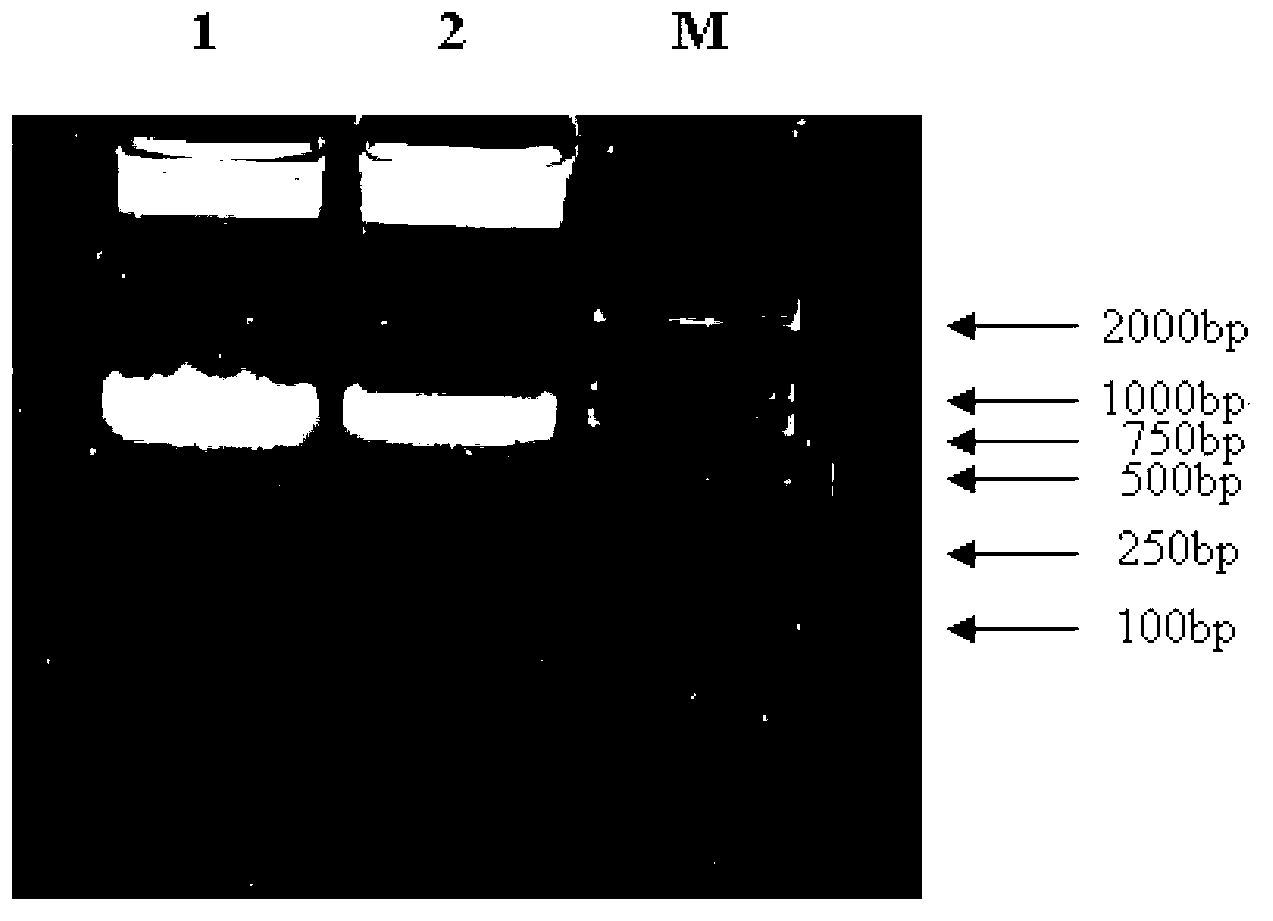
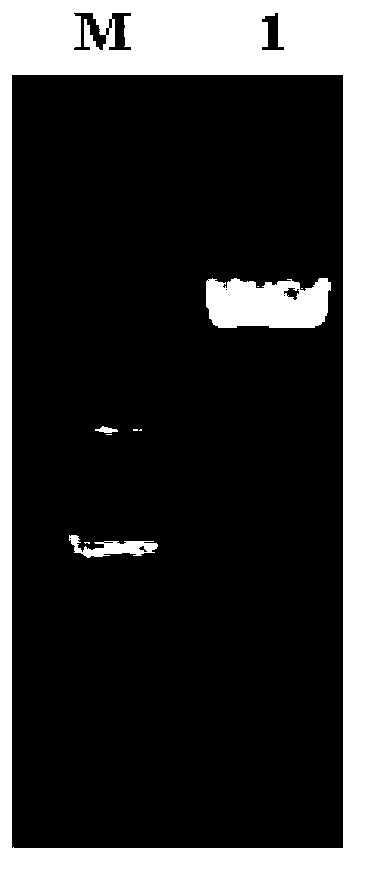
[0037] Total RNA was extracted from Agkistrodon halys in Jiangsu and Zhejiang provinces, and the VK gene was amplified by RT-PCR. Use 1% agarose gel electrophoresis to detect and recover the target band, then connect to the pGEM-T vector overnight at 4°C with T4 DNA ligase, and then transform into Escherichia coli DH5α; the transformant is first screened by blue and white, and then amplified by PCR (Amplification program: pre-denaturation at 94°C for 5 min; denaturation at 98°C for 10 s, annealing at 60°C for 15 s, extension at 72°C for 1 min, 30 cycles; extension at 72°C for 10 min, end of the reaction) and sequence identification. ( figure 1 )
Embodiment 2
[0038] The construction of embodiment 2 expression vector and the transformation of escherichia coli
[0039] Design upstream primer 5'- CCATGG TCATTGGAGGTGATGAATGTAACATA-3' (underlined part is Nco I restriction site) and downstream primer 5'- GCGGCCGCTCACGGGGGGCATGTCA-3' (the underlined part is the NotI restriction site), amplified by PCR method to obtain the VK gene without signal peptide. After the PCR product was digested by Nco I and NotI, it was inserted into the NcoI and NotI sites of the expression vector pET30a, and the plasmid was transformed into Escherichia coli DH5α, and the plasmid of the transformant was extracted and digested by NcoI and NotI. After sequencing and identification ( figure 2 ), into Escherichia coli BL21(DE3).
Embodiment 3
[0040] Induced expression of embodiment 3 recombinant escherichia coli
[0041] (1) Pick the positive single clone of recombinant Escherichia coli containing the plasmid pET30a / VK and add it to 5 mL LB liquid medium containing Kana antibiotics, culture with shaking at 37°C to make it OD 600 The value reaches 0.5~0.6;
[0042] (2) Take 0.5 mL of bacterial liquid, and centrifuge at 10,000 rpm to precipitate bacterial cells. Add 100 μL of 1×SDS gel loading buffer to the cells, and let it cool naturally in a boiling water bath for 10 minutes. This is the uninduced negative control;
[0043] (3) Add IPTG to the remaining 4.5 mL of the bacterial liquid to make the final concentration reach 1 mM, continue the induction culture for 4 h, take 0.5 mL of the bacterial liquid every 1 h, and centrifuge at 10,000 rpm to precipitate the bacterial cells. Example 4 Broken cells extract supernatant
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com