Antibody m19 of O-type foot-and-mouth disease virus structural protein as well as preparation method and application of antibody m19
A foot-and-mouth disease virus and structural protein technology, which is applied in the field of antibodies to the O-type foot-and-mouth disease virus structural protein, can solve problems such as complex antigen structure, scarcity of monoclonal antibodies, and obstacles to detection methods, and achieve the effect of improving sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 monoclonal antibody m19
[0038] 1. Preparation of hybridoma cells
[0039] 1.1 Immunization of mice
[0040] 5-6 week-old Balb / c mice were immunized with type O foot-and-mouth disease virus (O / Mya98) 146s as the antigen. The immunization method was multi-point subcutaneous injection on the back, and the injection volume was 100 μg per mouse. Freund's complete adjuvant was used for the first immunization, and Freund's incomplete adjuvant was used for subsequent booster immunizations. The interval between each immunization was two weeks. The mouse serum was collected one week after the third immunization to detect the antibody titer. When the antibody titer is not low At 1:1440, impulse immunization was performed, that is, the antigen was injected intraperitoneally, and three days after the impulse immunization, the spleen of the mouse was taken for fusion with myeloma cells.
[0041] 1.2 Cell Fusion
[0042] Spleen cells of immunized m...
Embodiment 2
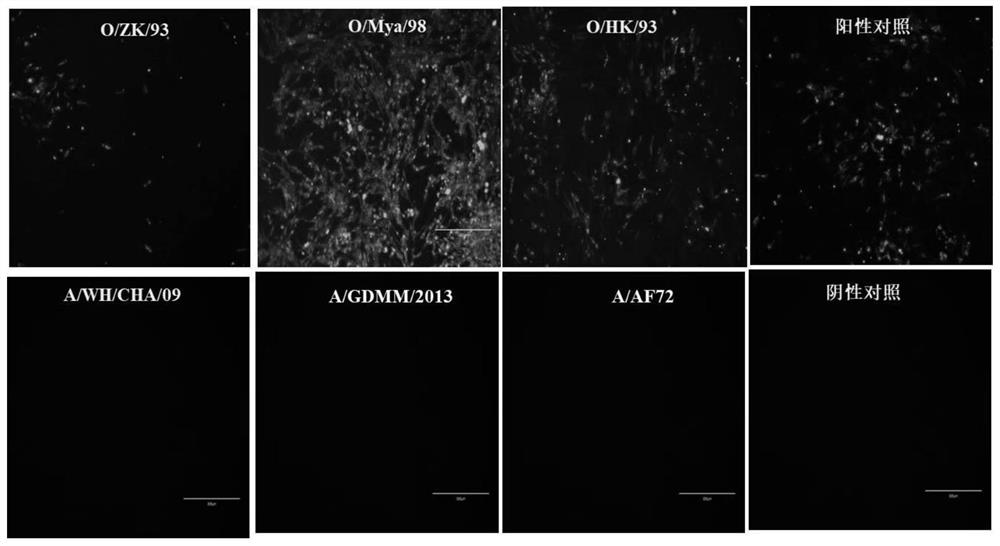
[0061] Embodiment 2 monoclonal antibody m19 reacts with different lineage foot-and-mouth disease virus antigens
[0062] 1. Indirect immunofluorescence test:
[0063] Representative strains of different lineages of type O foot-and-mouth disease (O / ZK / 93, O / Mya / 98 and O / HK / 93 strains) and representative strains of different lineages of type A foot-and-mouth disease (A / AF72, A / GDMM / 2013 and A / WH / CHA / 09) (both strains are preserved by the National Reference Laboratory for Foot-and-Mouth Disease) to infect BHK-21 cells, and the reaction between the prepared monoclonal antibody m19 and the FMDV antigen was determined by the fluorescence intensity under the fluorescence microscope. The specific operation steps are as follows:
[0064] (1) Cell culture: spread BHK-21 cells evenly in a 12-well cell culture plate, and inoculate the cells after the cells grow to about 70%;
[0065] (2) Inoculation: inoculate 3 lineages of type O virus strains and 3 lineages of type A virus strains in...
Embodiment 3
[0072] Example 3 Establishment and application of solid-phase competitive ELISA detection method for O-type foot-and-mouth disease virus structural protein
[0073] 1. Determination of the optimal reaction conditions for solid-phase competitive ELISA detection method of O-type foot-and-mouth disease virus structural protein
[0074] 1.1 Determination of the optimal concentration of rabbit antiserum and O-type FMD virus inactivated antigen for structural protein of O-type foot-and-mouth disease virus:
[0075] The optimal concentration of rabbit antiserum and inactivated antigen of type O foot-and-mouth disease virus (preserved in our laboratory) was determined by square array titration. First, use Na 2 CO 3 / NaHCO 3 The coating solution (pH=9.6) was serially diluted from 1:250 in the dilution plate to the O-type foot-and-mouth disease virus structural protein rabbit antiserum (horizontal), and correspondingly transferred into the enzyme plate (50 μ L / well), 4 Overnight at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com