Polypeptide preparations
A peptide and amino acid technology, applied in the direction of peptides, peptide sources, peptides, etc., can solve problems such as infection or disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Peptide-HLA-I complex formation
[0035] The experiment was carried out according to the Monomer / Tetramer production protocol experimental operation flow of the easYmer HLA-A*02:01 MHC Tetramers Kit kit of the immunoware manufacturer.
[0036] 1. Weigh an appropriate amount of peptide to be tested (see Table 1), and dissolve it in dimethyl sulfoxide to 1 mM.
[0037] 2. The positive control peptide (the positive control peptide is provided by the immunoware easYmer HLA-A*02:01 MHCTetramers Kit), and the peptide to be tested is diluted to 25 μM with double distilled water.
[0038] 3. Prepare each reaction tube on ice according to the following table (see Table 2), and mix well by repeatedly pipetting with a pipette to avoid the generation of air bubbles.
[0039] 4. After sealing with the sealing tape, incubate at 18 degrees for 48 hours before use.
[0040] no size sequence no size sequence 1# 1,380 GVAPGTAVLRQW 19# 1,380 RTV...
Embodiment 2
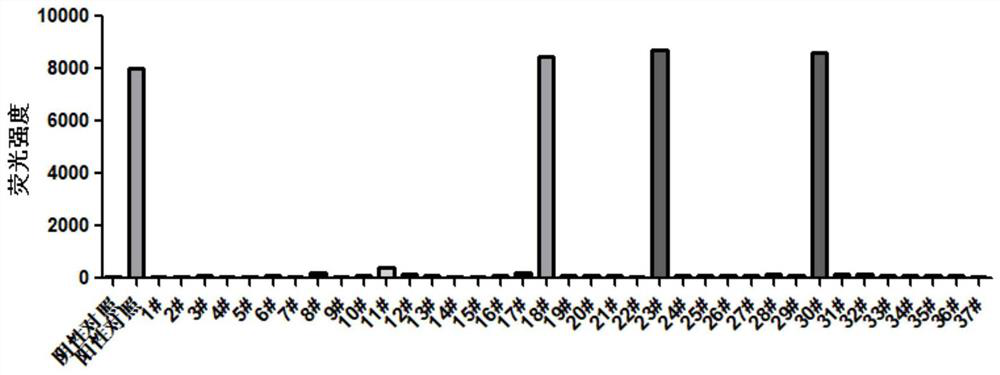
[0044] Example 2: Detection of the Formation of Peptide-HLA-I Complex Based on Flow Cytometry
[0045] 1. Add 4 μl of peptide-HLA-I complex solution to 46 μl of dilution buffer and mix well.
[0046] 2. Add 25 μl peptide-HLA-I complex dilution to 50 μl dilution buffer and mix well.
[0047] 3. Transfer 40 μl of the dilution from step 2 to a new EP tube.
[0048] 4. Streptavidin-coated magnetic beads (6-8 μm), diluted 45 times with dilution buffer, added 20 μl to each tube, and mixed well.
[0049] 5. Incubate on a shaker at 37 degrees for 1 hour.
[0050] 6. Add 160 μl FACS solution to each tube.
[0051] 7. Centrifuge at 700g for 3 minutes and discard the supernatant.
[0052] 8. Resuspend in 200μl FACS solution, centrifuge at 700g for 3min, discard the supernatant, repeat this step, and wash twice.
[0053] 9. Dilute PE-labeled anti-human monoclonal antibody BBM.1 200 times with FACS solution.
[0054] 10. Add 50 μl of antibody diluent to each tube to resuspend, and in...
Embodiment 3
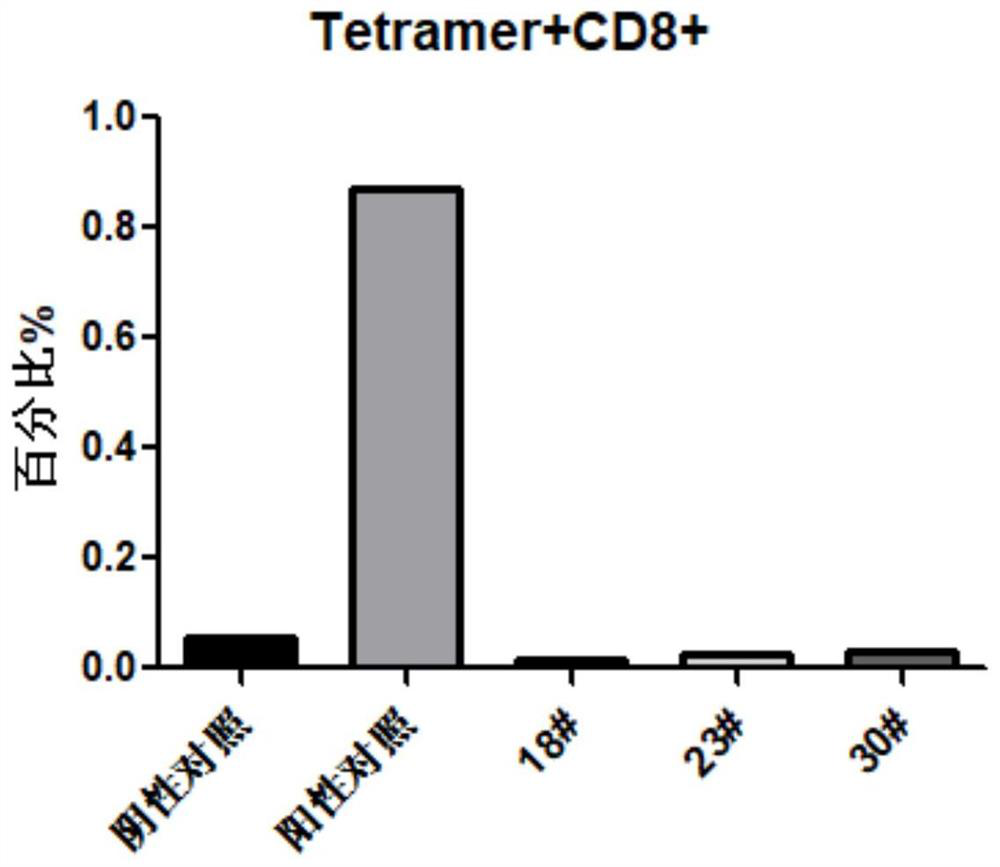
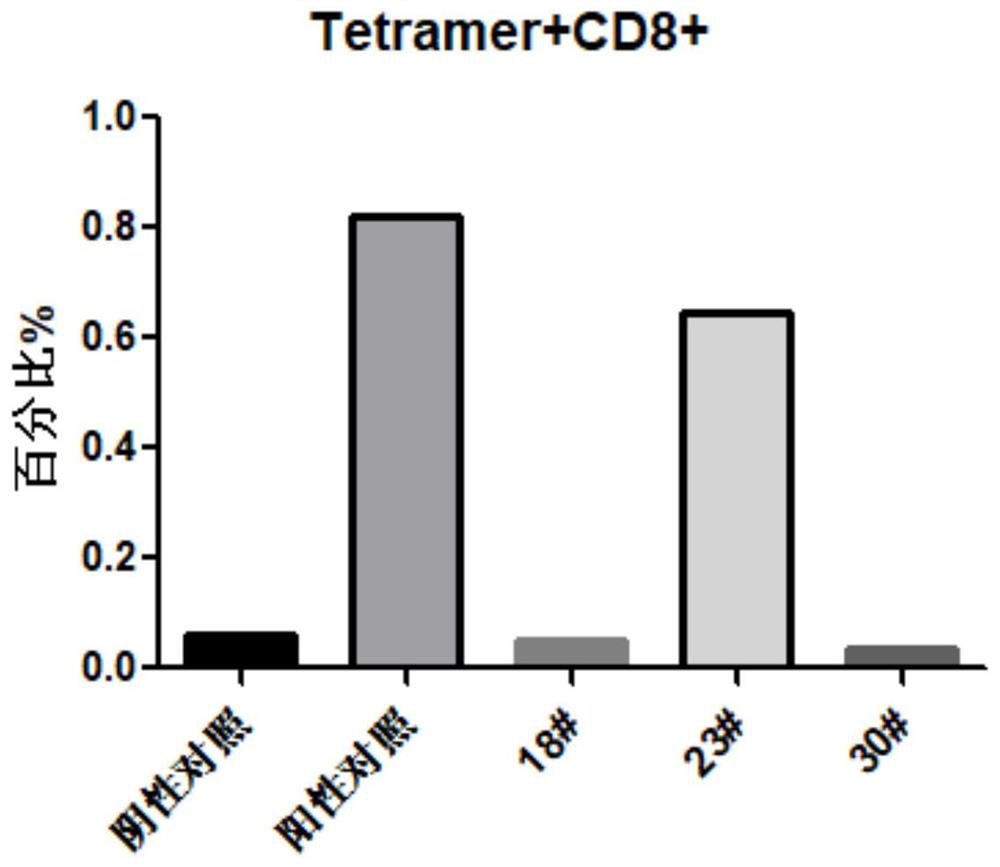
[0059] Example 3: Tetramer related:
[0060] This experiment was carried out in accordance with the Monomer / Tetramer production protocol experimental procedure of the easYmer HLA-A*02:01 MHC Tetramers Kit kit from the immunoware manufacturer.
[0061] Preparation of tetramers.
[0062] 1. Take 6 μL of the prepared 500nM polypeptide 18#, 23#, 30# and HLA-I monomer complex in a 0.2mL centrifuge tube, add 0.48μL Streptavidin-BV421 (BD; Cat#563259; 0.1mg / ml ).
[0063] 2. After mixing evenly, incubate at 4°C in the dark for 1 hour.
[0064] Isolation of Human Peripheral Blood Mononuclear Cells (PBMC)
[0065] 1. Take human venous anticoagulated whole blood (EDTA) and dilute it with an equal volume of normal saline.
[0066] 2. Add an appropriate amount of lymphocyte separation medium into a 15mL centrifuge tube, spread the diluted blood on top of the separation medium, and keep the interface between the two liquid surfaces clear.
[0067] 3. At room temperature, centrifuge ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com