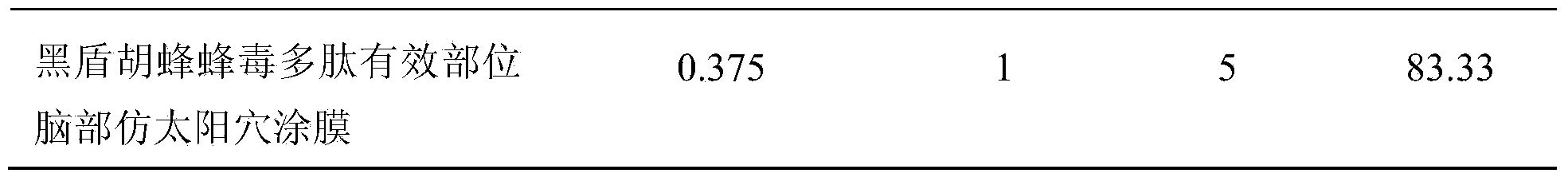Patents
Literature
95 results about "Biological toxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biological toxins consist of any toxic substance produced by microorganisms, plants, or animals. They include metabolites of living organisms, degradation products of nonliving organisms, and those materials rendered toxic by the metabolic activity of microorganisms.
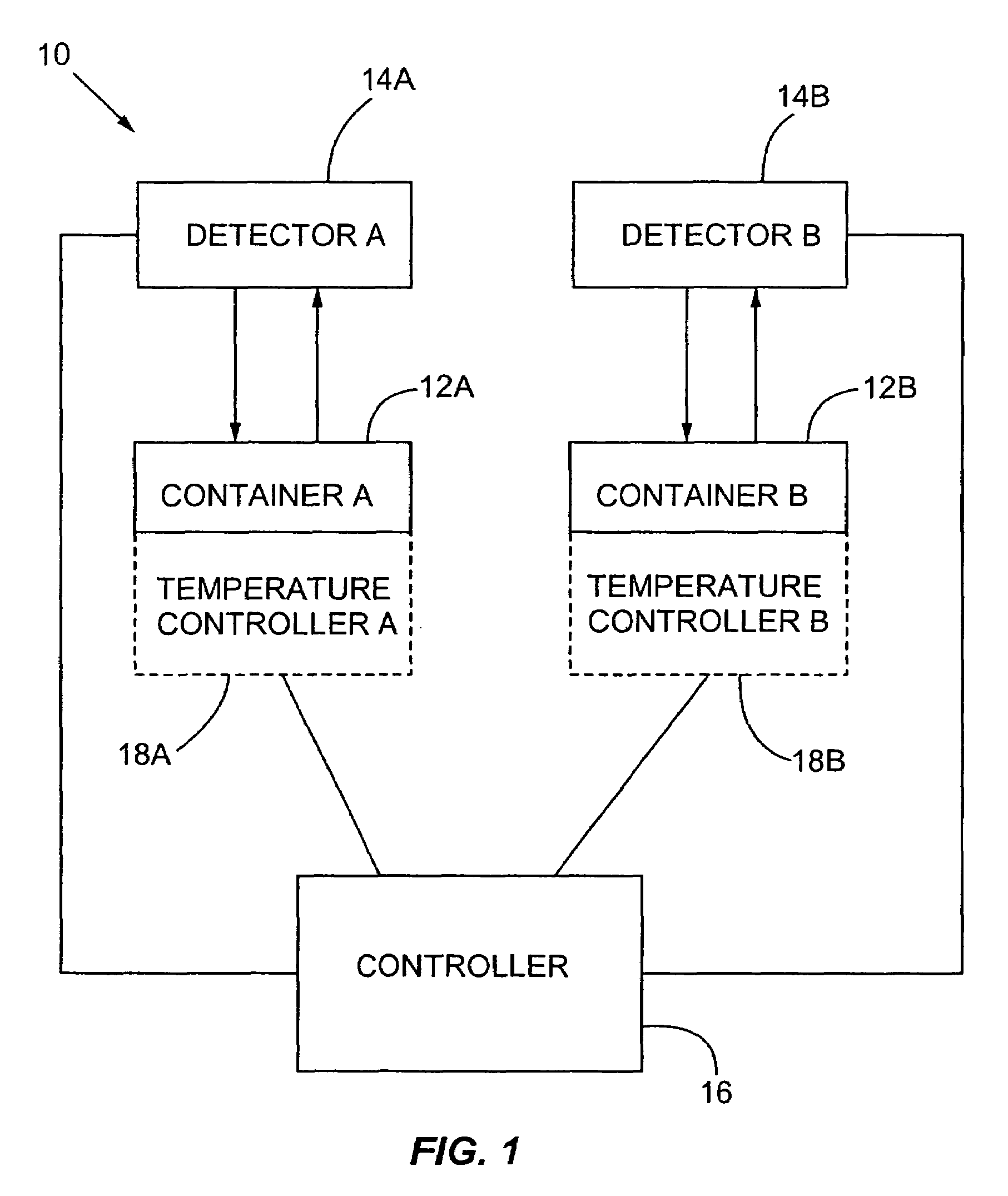
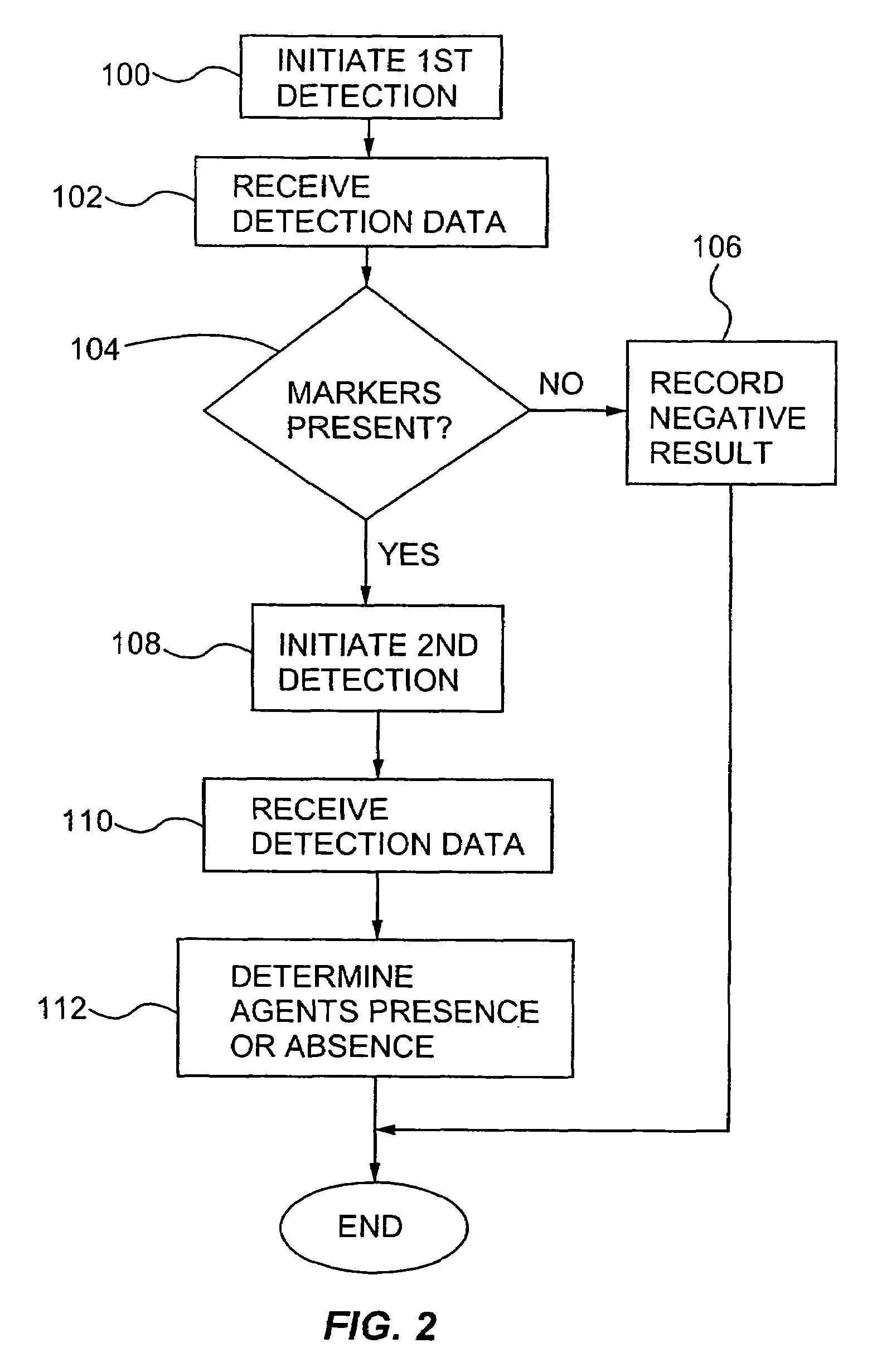
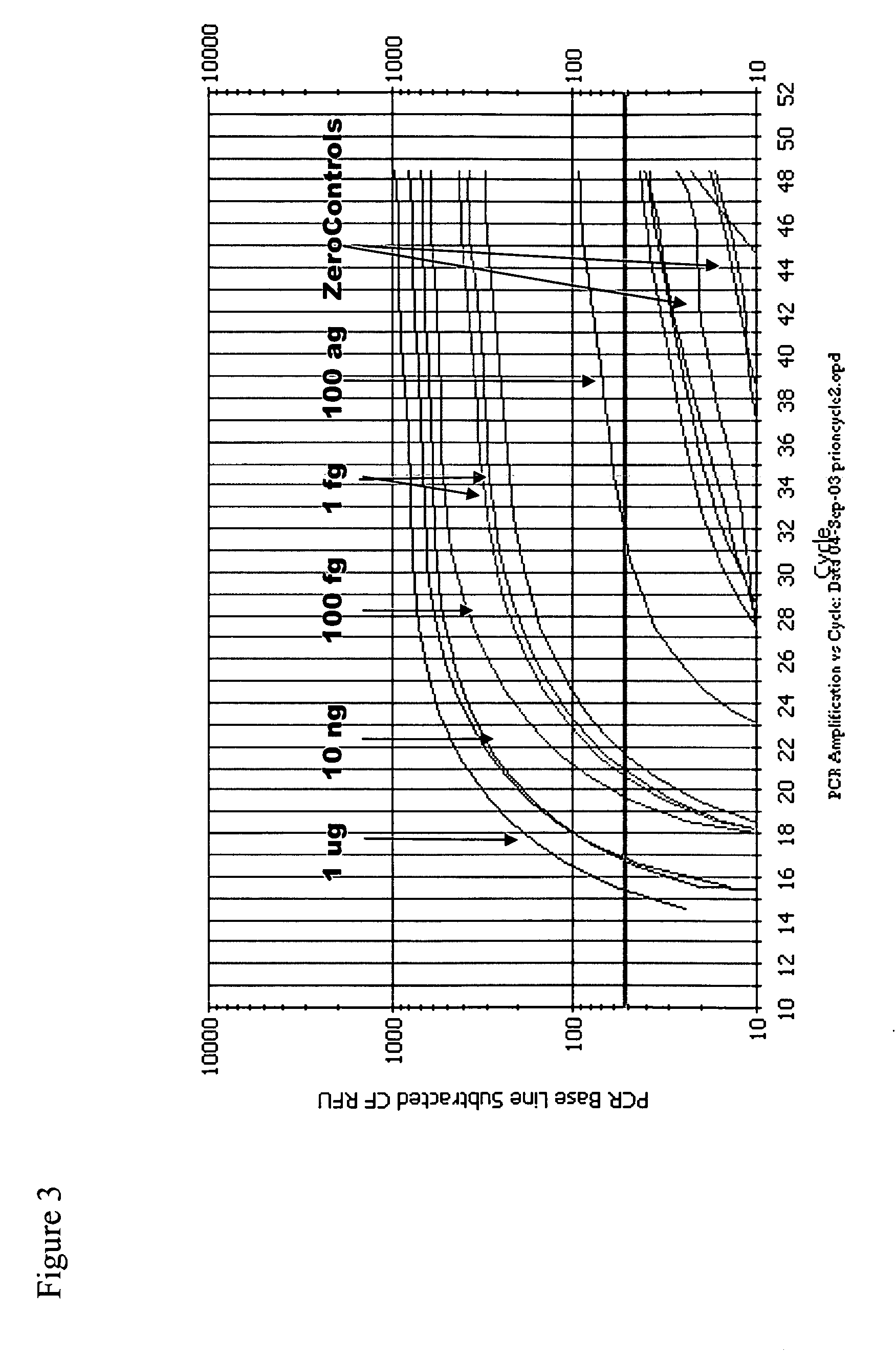
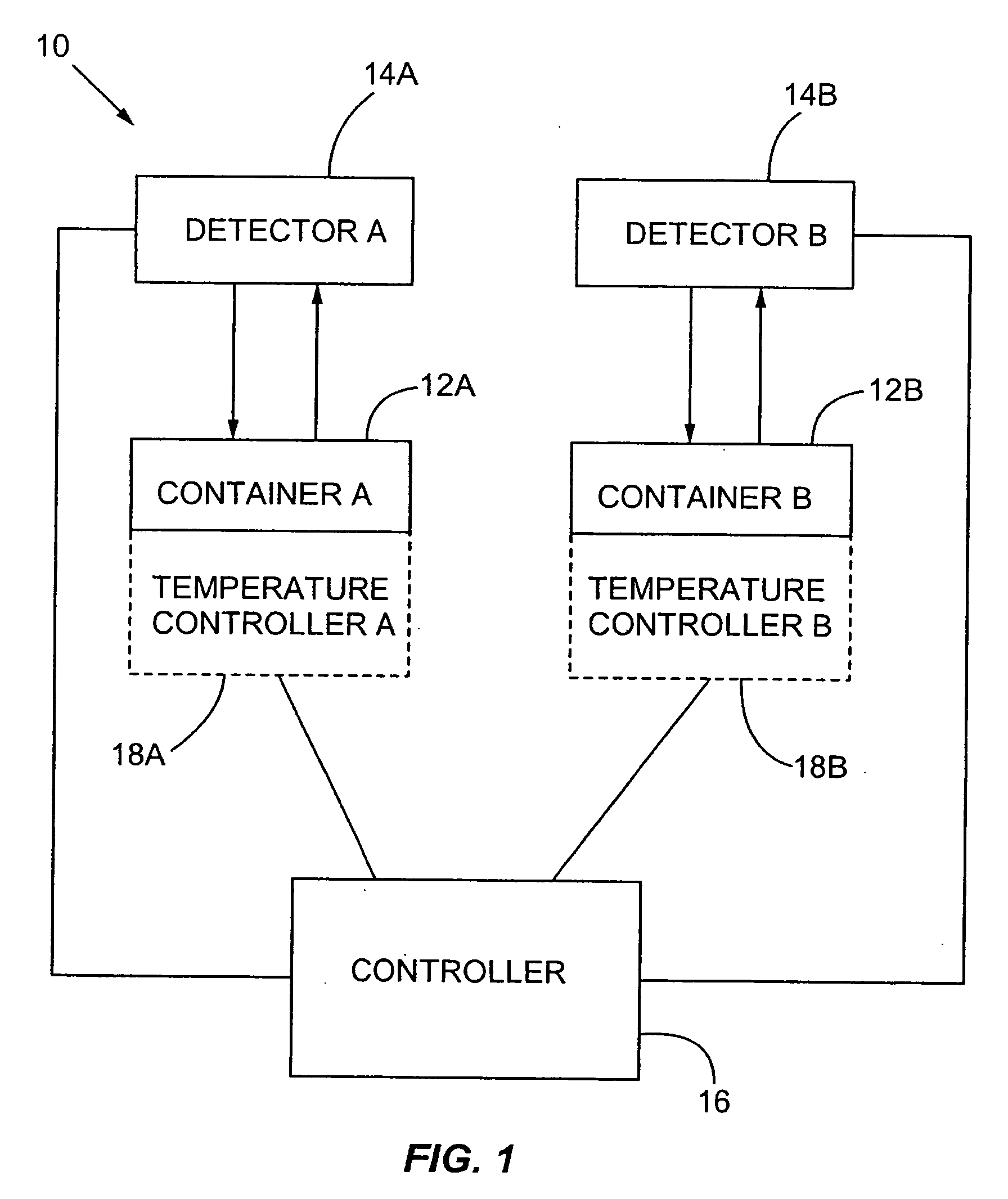
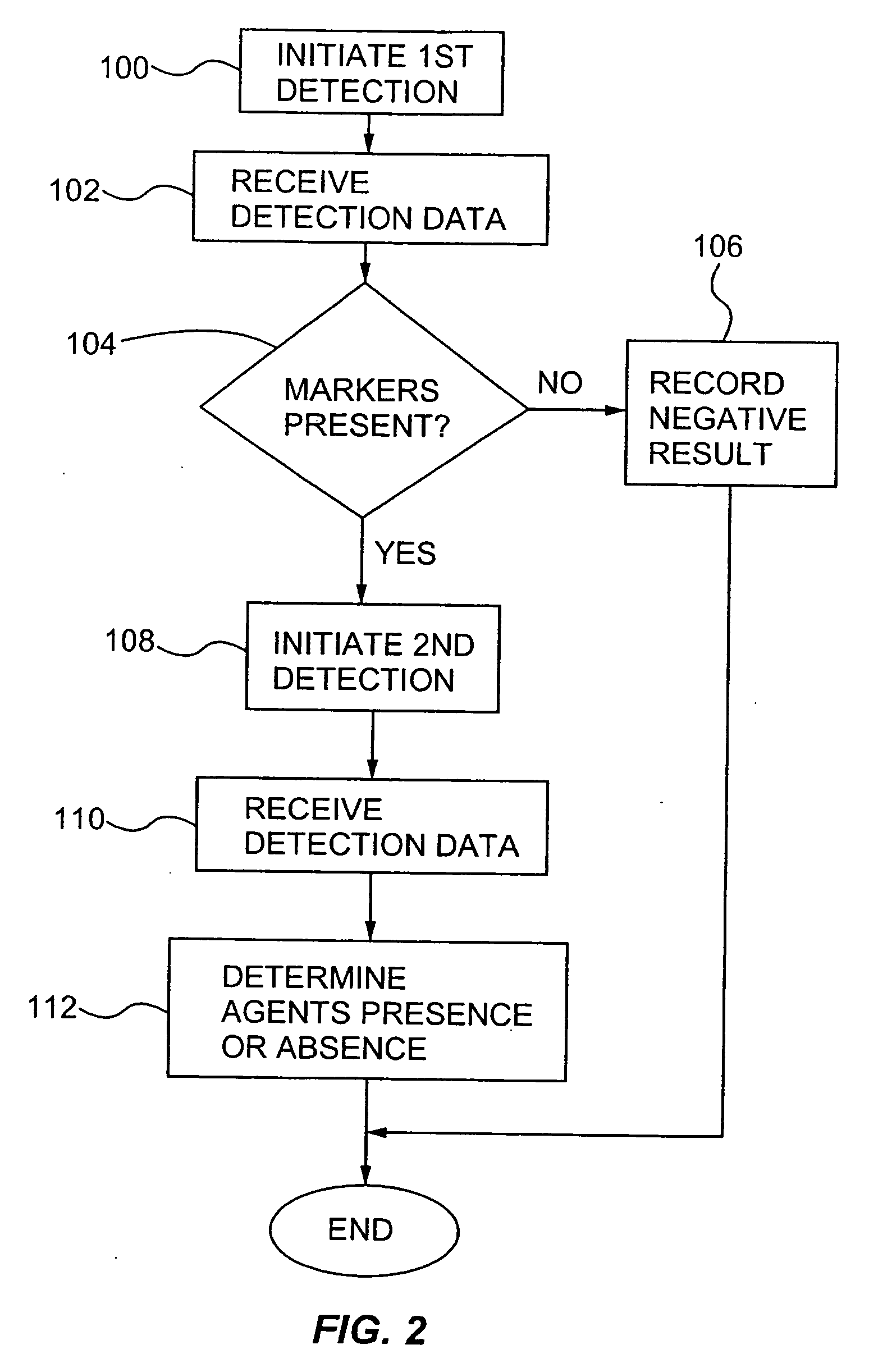
Multiplexed detection of biological agents
Described are methods, kits and systems for multiplexed detection of biological agents in a sample, e.g., multiplexed detection of e.g., bacteria, viruses, and biological toxins. The method utilizes two markers for each agent; the presence or absence in a sample of each of the two markers per agent is determined in separate reactions; however each reaction is used to detect a single marker for multiple agents. Also disclosed is the multiplexed detection method using real time PCR. The invention provides an efficient, cost-effective, and specific method for multiplexed detection of biological agents.
Owner:CEPHEID INC
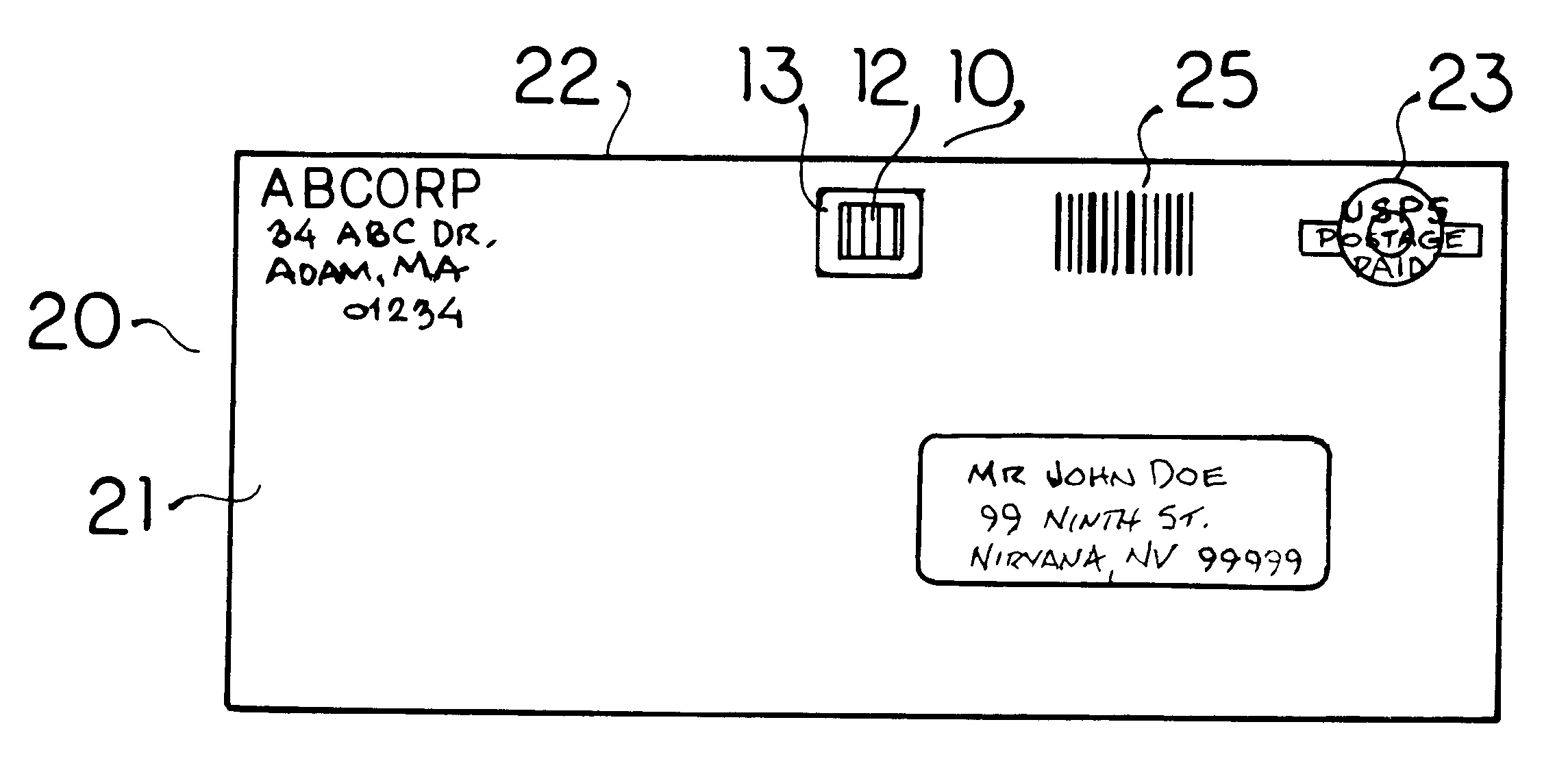
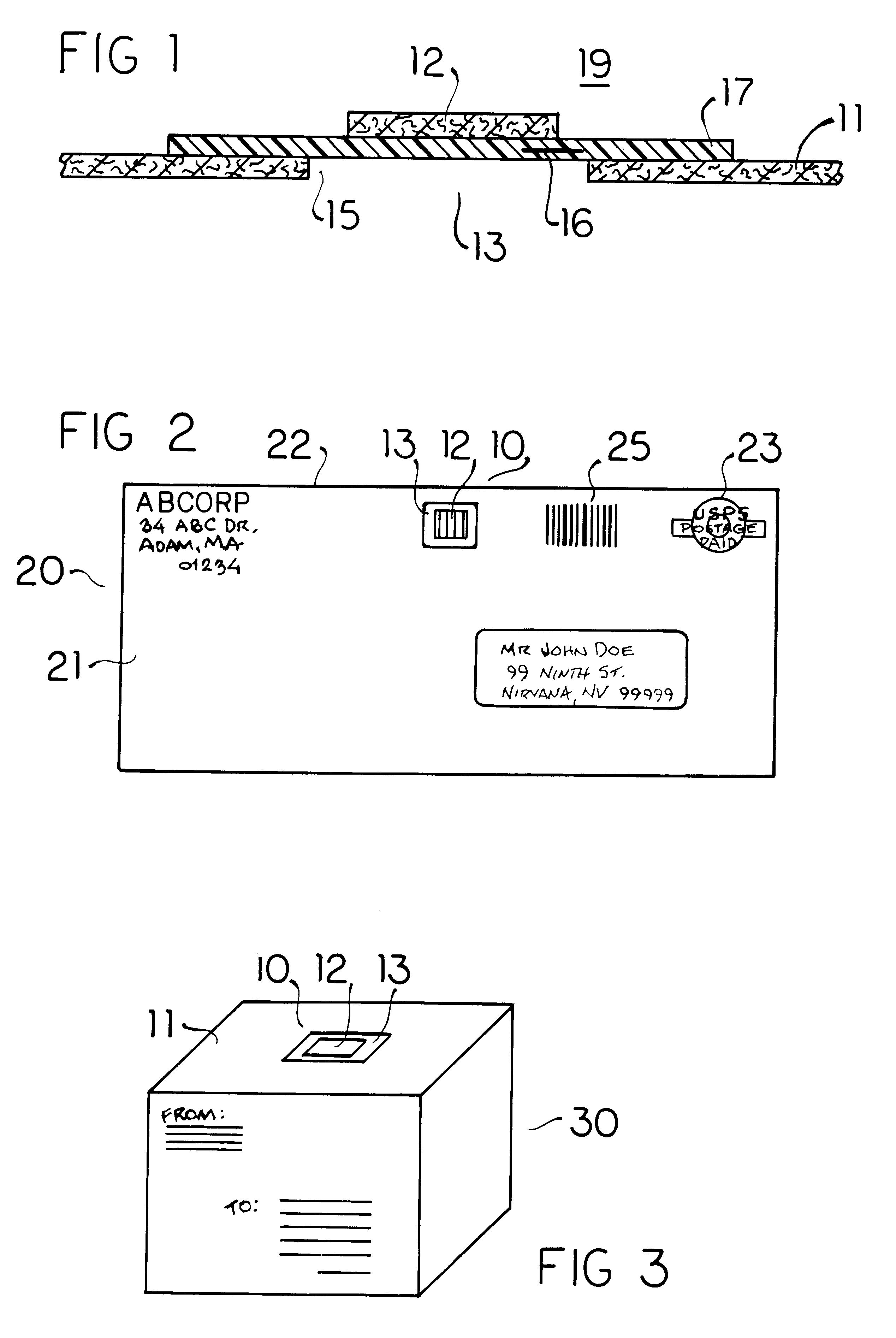
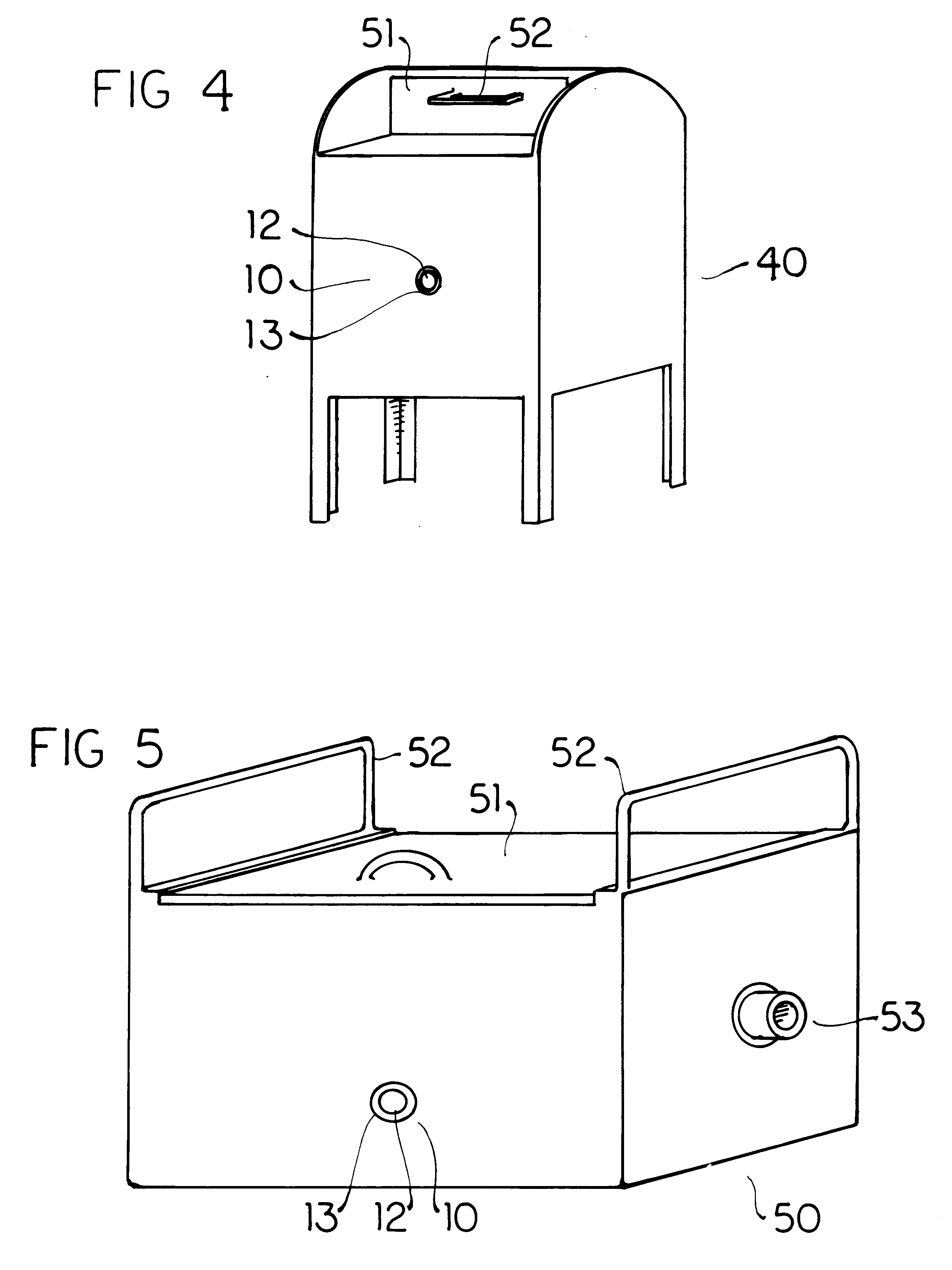
Biological toxin detection system for mailed materials
InactiveUS6524846B1Easy to manufactureReadily adheres to paperBioreactor/fermenter combinationsBiological substance pretreatmentsPorous substrateQuarantine
Envelopes and other containers intended for mail transportation possess a biological agent / toxin indicator operative upon the interior in detection of volatile bases including gaseous amines released from bacterial biological agents including Bacillus antracis, i.e. anthrax. The biological agent indicator has an acidic acid-base indicator compound that irreversibly changes color when neutralized by volatile bases. The compound is applied in liquid form to an appropriately porous substrate. A polymeric matrix is specifically suggested for this substrate. Irreversible indication of the presence of volatile bases including amines produced by live bacterial agents as toxins at temperate ambient conditions down to below freezing is provided. An indicator compound having a pH of 2-5 is recommended. Halogenated xanthene, sulphonated azo, and sulphonated hydroxy-functional triphenylmethane dyes are suggested. The presence of toxins produced by live bacterial biological agents within a package or container upon which the indicator is mounted is indicated by a slowly reversible color change. Primary public use envelopes and collection containers, secondary transportation and stationary quarantine enclosures, and articles worn inside a room with mail and when handling mail including a badge and gloves are specifically suggested.
Owner:ROBINSON JR WILLIAM L
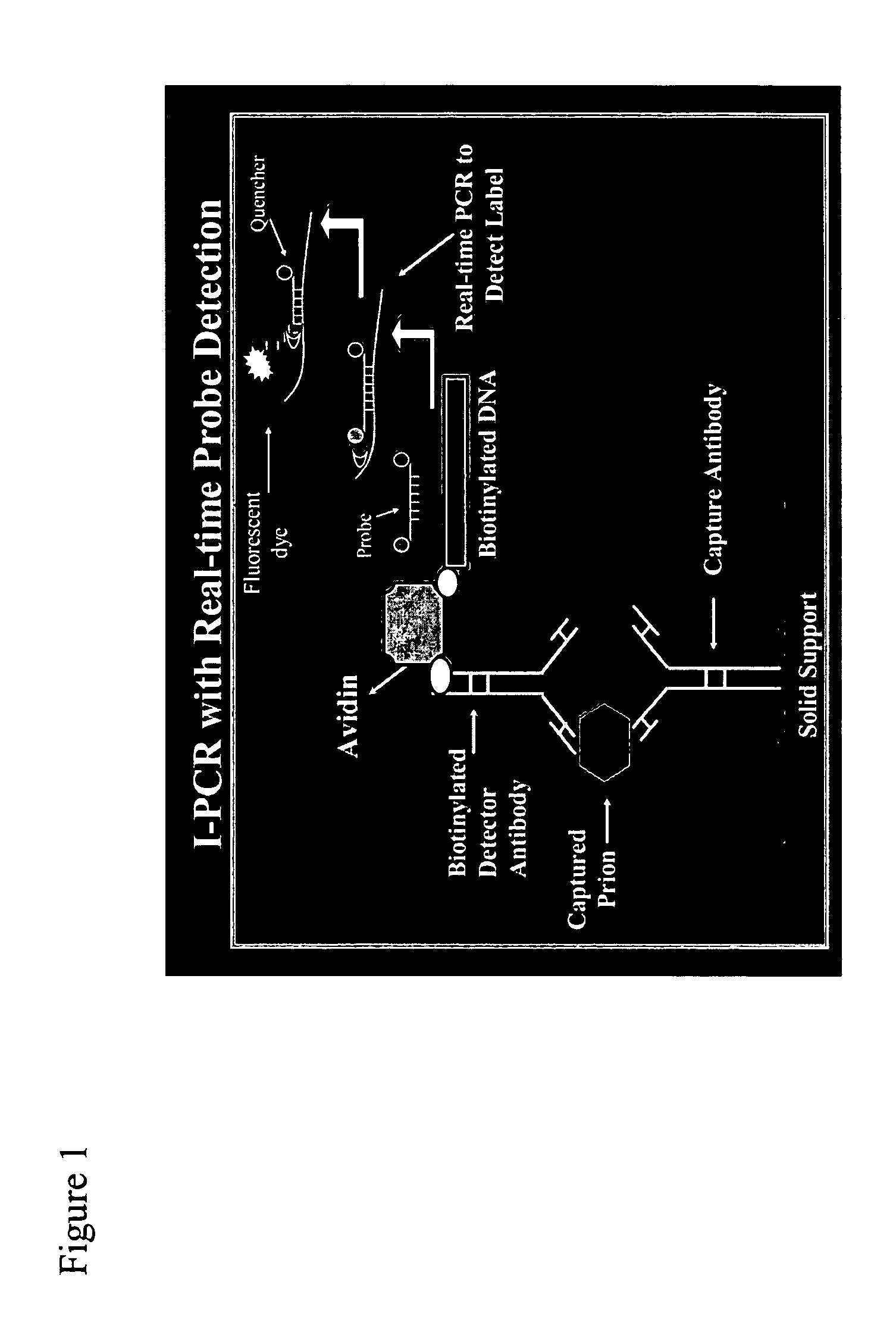
Immuno-PCR method for the detection of a biomolecule in a test sample
InactiveUS20050239108A1Simple and highly sensitiveSimple and highly methodMicrobiological testing/measurementAssay labelsHuman bodyNucleic acid amplification technique
The invention relates to methods and kits for detecting and / or monitoring biological molecules in a test sample. For example, the invention relates to methods and kits for detecting and / or monitoring HIV p24 antigen in human body fluid, biological toxins such as ricin or botulism in an environmental or biological sample, and prion protein from human, deer or bovine, such as PrPSC, in a biological sample. The antigen detection signal is boosted by amplification of a polynucleotide linked to a detector molecule using methods for nucleic acid amplification technology.
Owner:UNIV OF MARLAND BALTIMORE +1
Multiplexed detection of biological agents
ActiveUS20050250146A1Sugar derivativesMicrobiological testing/measurementBiochemistryBiological toxin
Described are methods, kits and systems for multiplexed detection of biological agents in a sample, e.g., multiplexed detection of e.g., bacteria, viruses, and biological toxins. The method utilizes two markers for each agent; the presence or absence in a sample of each of the two markers per agent is determined in separate reactions; however each reaction is used to detect a single marker for multiple agents. Also disclosed is the multiplexed detection method using real time PCR. The invention provides an efficient, cost-effective, and specific method for multiplexed detection of biological agents.
Owner:CEPHEID INC
Extracellular matrix materials as vaccine adjuvants for diseases associated with infectious pathogens or toxins
Disclosed are vaccines and vaccine adjuvants useful in the treatment and / or prevention of infection and diseases associated with infectious pathogens, such as tetanus, as well as diseases associated with biological toxins. Also provided are methods of preparing an adjuvant and the vaccine containing the adjuvant. Methods are also provided for vaccinating / immunizing an animal against infection and diseases associated with infectious pathogens, such as tetanus, and other diseases associated with biological toxins. Adjuvant materials are presented that are prepared from an extracellular matrix material. The adjuvants are demonstrated to enhance the immunogencity of an infectious pathogen antigen or biological toxin antigen of interest, as well as to enhance the survival of an immunized animal.
Owner:UNIV OF NOTRE DAME DU LAC +1
Electrochemical sidestream immune quantitative test paper sensor based on microgap array electrode and method thereof for detecting biotoxin
InactiveCN101509924AEasy to makeLow costComponent separationMaterial analysis by electric/magnetic meansFumonisin B1Ochratoxin A
The invention relates to an electrochemical lateral-flow immunity quantitative test paper sensor based on microclearance array electrode, and a method thereof used for detecting biological toxins such as ochratoxin A and fumonisin B1. The sensor includes two parts, namely, immune chromatographic analysis test paper strip and electrochemical detecting part. The fast detecting test paper sensor has strong specificity, can realize quantitative detection, can be used at the temperature between 4 and 40 DEG C, and the result can be observed after ten minutes, thus being suitable for units or individuals to quickly detect ochratoxin A and fumonisin B1 in animal derived food samples, and being expected to become an effective technical means for the field screening of ochratoxin A and fumonisin B1 in food and feed samples.
Owner:湖南省宜生科技有限公司 +1
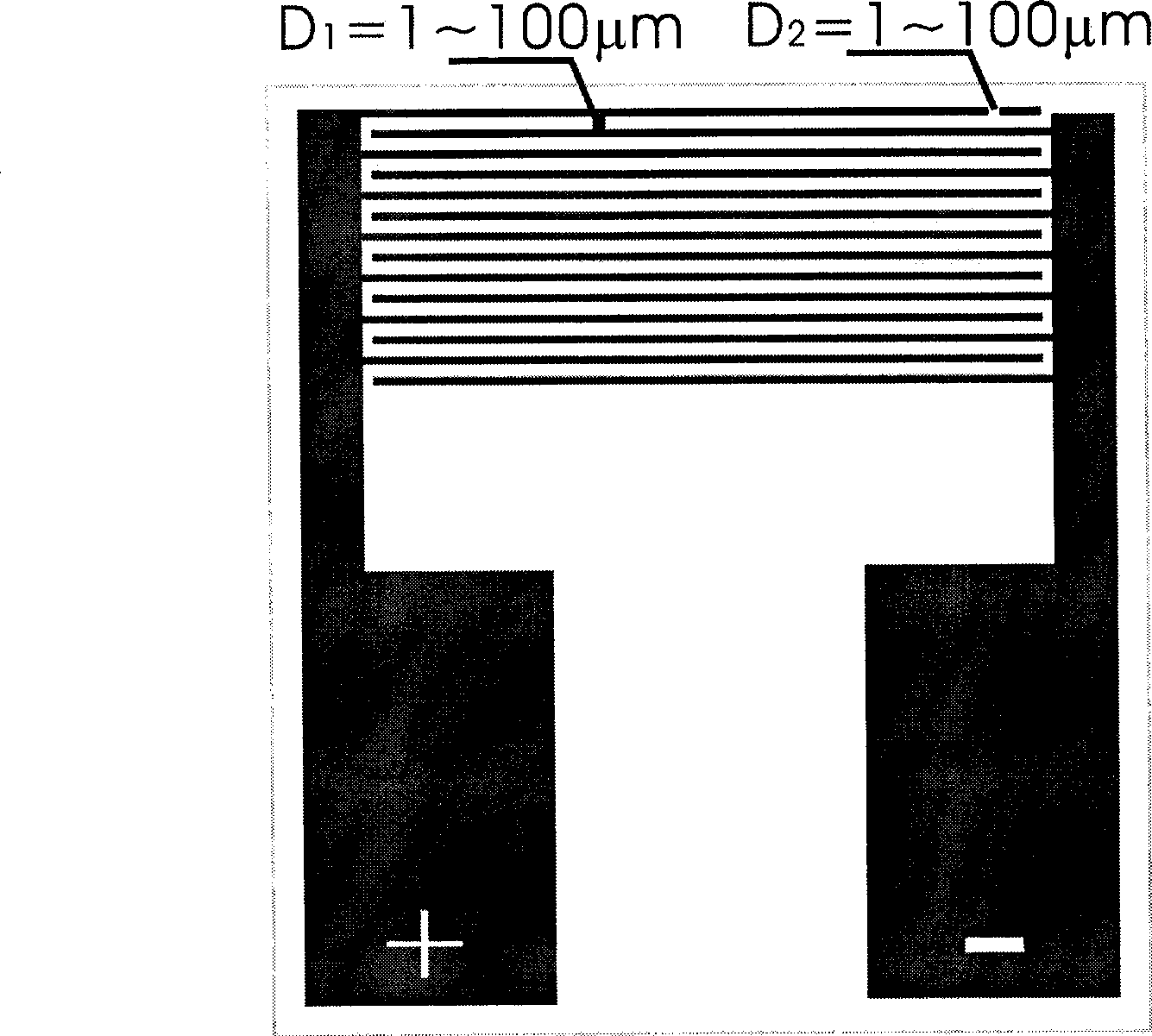
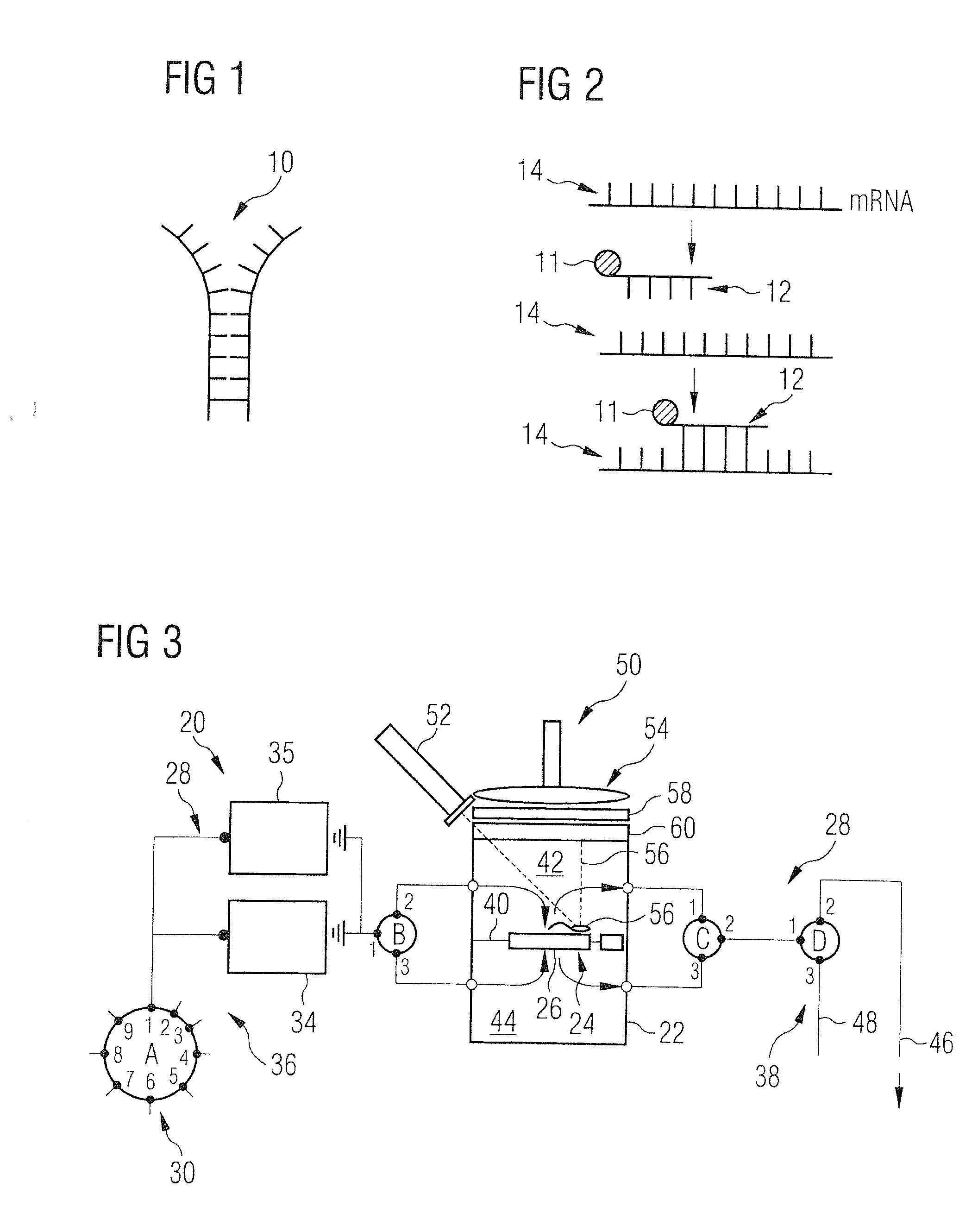
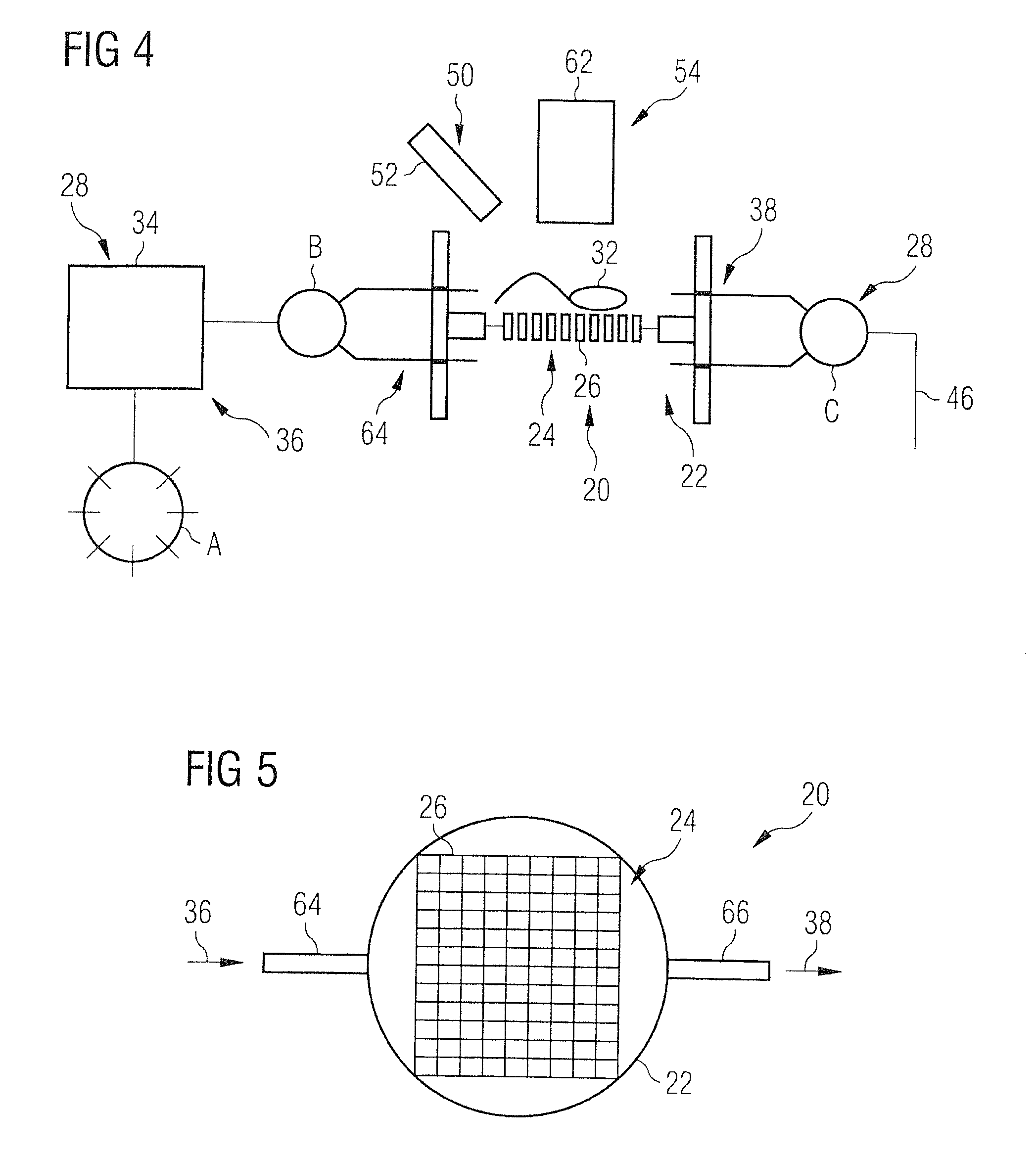
Detection device for detecting biological microparticles such as bacteria, viruses, spores, pollen or biological toxins, and detection method
ActiveUS20100136556A1Rapid and reliable and uncomplicated automatic detectionRapid and reliable and detectionBioreactor/fermenter combinationsBiological substance pretreatmentsBacterial virusSpore
A device for the detection of micro particles that can be marked by probes or antibodies capable of being detected by radiation has a filter, a supply system, and a detection system. Fluid to be examined is passed over a filter to filter out the micro particles and to perform the marking steps by supplying corresponding marking substances to the filter.
Owner:EADS DEUT GMBH
Method and apparatus for sterilizing and disinfecting air and surfaces and protecting a zone from external microbial contamination
ActiveUS20170304472A1Short action timeEffective treatmentFouling preventionFruits/vegetable preservation by irradiation/electric treatmentParticulatesToxic material
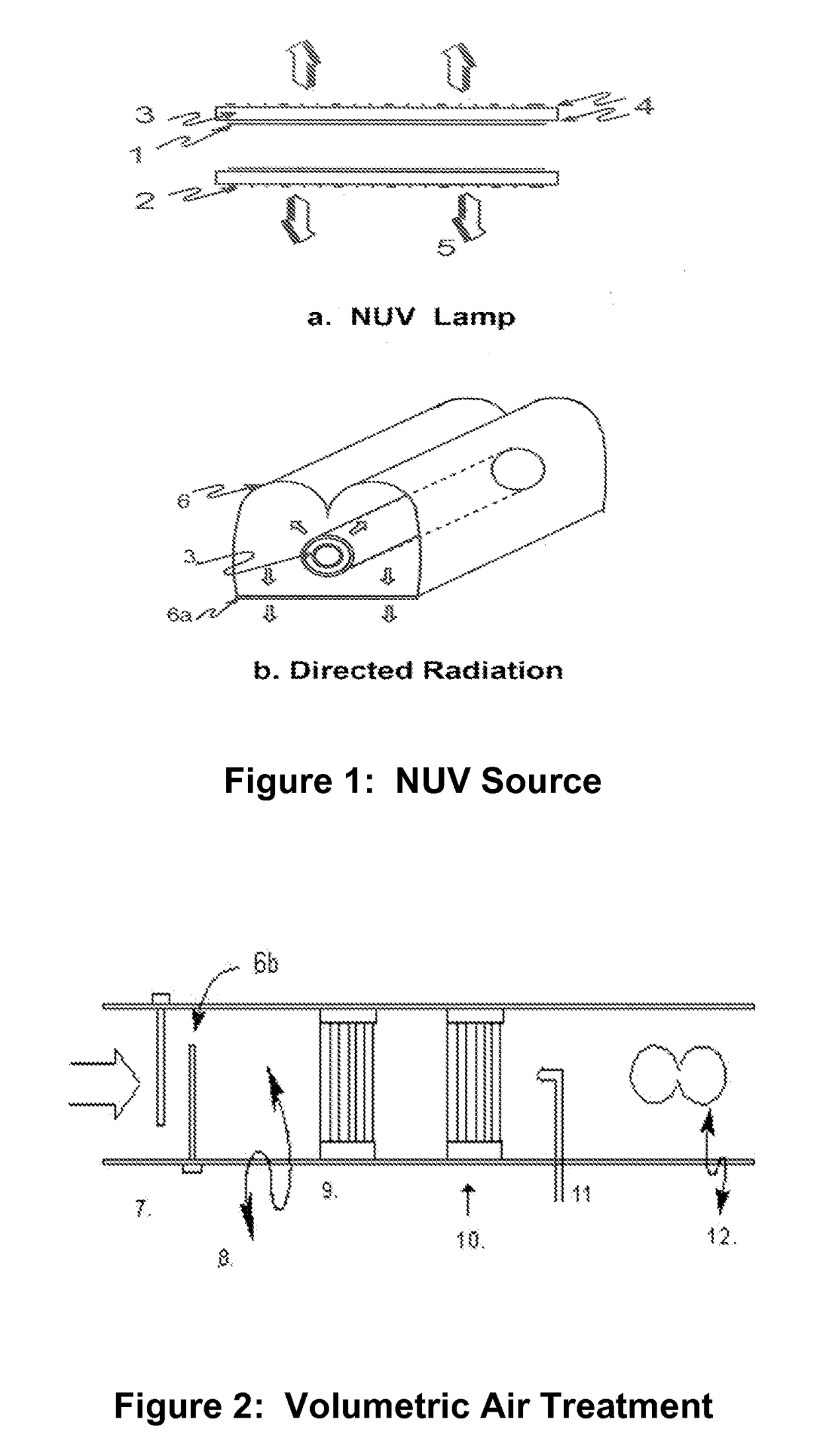
This invention relates to a method, process and apparatus for disinfecting and sterilizing all types of surfaces contaminated with microorganisms and toxic substances to render both inactive. Furthermore, this invention relates to both a method and apparatus for disinfecting and / or sterilizing breathable air and then using this air to protect a confined space from external contamination. The apparatus consists of a new ultra-violet (NUV) source that is more effective than mercury based 254 nm light for destroying DNA of virus, bacteria, spores and cists. It is most effective in breaking chemical bonds in toxic gases and Biotoxins that are useful to terrorists. It is combined with other apparatus that remove particulates and byproducts sometimes produced by the NUV source and maintains positive pressure of the confined space so as to prevent the influx of air from outside the protected zone.
Owner:NEISTER S EDWARD
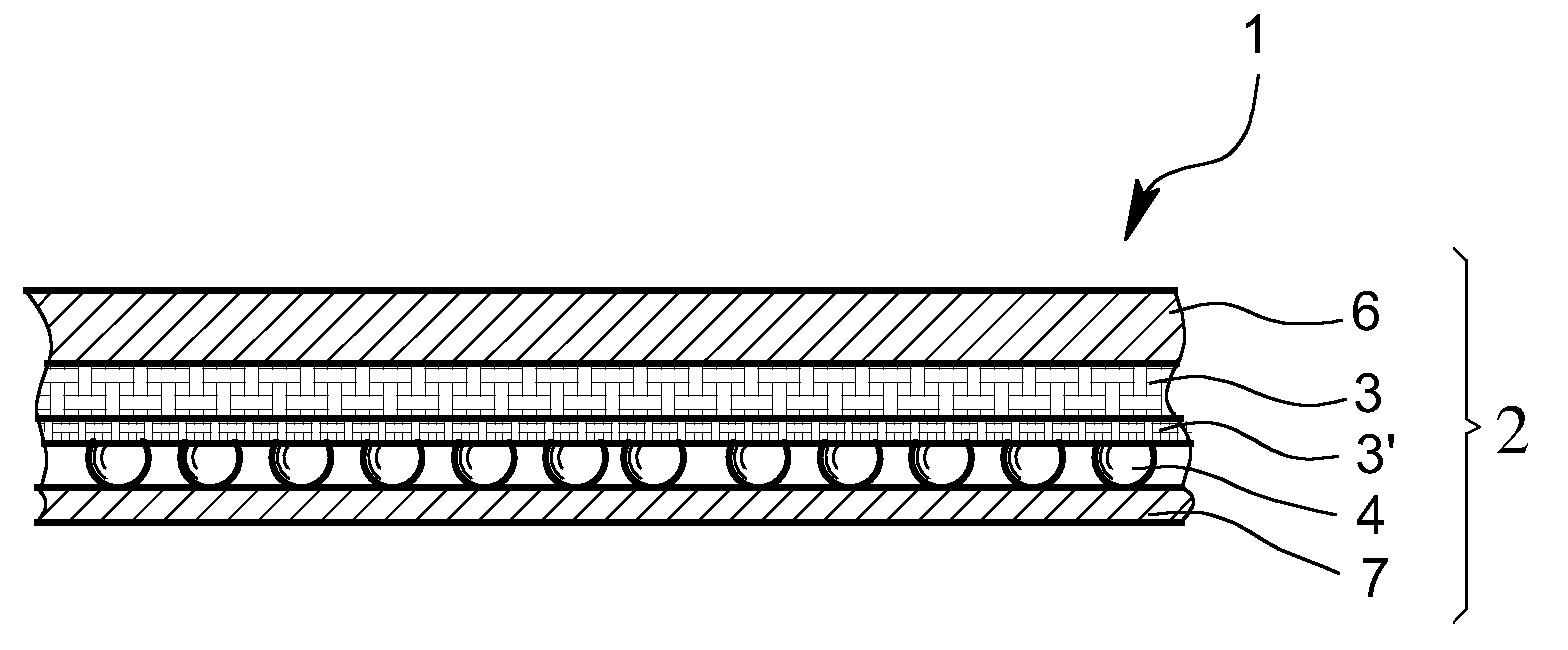
Textile Material Having Increased Mechanical Strength, in Particular Having Increased Resistance to Piercing or Shooting
ActiveUS20120128947A1Suitable for productionHigh wearing comfortChemical protectionArmourEngineeringBiological toxin
The present invention relates to a textile web material having improved mechanical strength, in particular having resistance to piercing and / or shooting, having a protective function against chemical and / or biological toxins, in particular weapon agents. The textile web material according to the invention comprises a multilayer construction, wherein the layer construction comprises at least one layer resistant to piercing and / or shooting, and at least one adsorption layer based on discrete adsorption particles.
Owner:BLUCHER GMBH
Biotoxin sorbent and method for preparing the same
InactiveUS20100189871A1Good compatibilityImprove efficiencyOther chemical processesAnimal feeding stuffDiseaseBiotechnology
The present invention provides a combination used for absorbing toxins in feed, comprising 1-10% by weight of clay and 90-99% by weight of yeast manna oligosaccharide, wherein the yeast manna oligosaccharide is extract of yeast cell wall which is separated from Saccharomyces cerevisiae. The biotoxin sorbent product can be used to feed any animals, including livestock, fowls, marine lives and ruminants. When mixing with feed, owing to its strong toxin adsorption capability, this absorbent may reduce the amount of toxins entering bodies of animals, thereby improving productivity and health of animals, reducing diseases caused by toxins.
Owner:ANGELYEAST CO LTD
Integrated method for high-throughput identification of novel pesticidal compositions and uses therefor
InactiveUS20130227743A1Elevated resistance of the organism to a pestBiocideBacteriaPlant cellNucleic acid sequencing
Methods to rapidly identify nucleic acid sequences encoding novel biotoxins are provided. Particularly, methods to rapidly sample and screen extrachromosomal genetic content of microorganisms for novel sequences of interest are described. Compositions comprising coding sequences for biotoxins, and polypeptides and uses derived therefrom are provided. Compositions and methods are useful, for example, for conferring pesticidal activity to bacteria, plants, plant cells, tissues, and seeds.
Owner:SYNTHETIC GENOMICS INC
Tilapia feed taking cotton seed meal and rapeseed meal as main protein materials
InactiveCN101822329AImprove stabilityToxic reductionFood processingAnimal feeding stuffAnimal scienceVeterinary Drugs
The invention provides a tilapia feed taking cotton seed meal and rapeseed meal as main protein materials, belonging to the technical field of fish feed. The tilapia feed is characterized by comprising the following components by weight percent: 10-26 percent of cotton seed meal, 30-50 percent of rapeseed meal, 0-20 percent of soybean meal, 15-40 percent of sub-powder, 0.1-1.5 percent of tangerine peel, 0.3-1.5 percent of dry leaves of eucommia bark, 0.5-4 percent of calcium hydrophosphate, 0.1-0.3 percent of compound vitamin, 0.1-0.5 percent of compound trace ore element, 0.1-0.5 percent of salt and 0.5-3 percent of compound premix. The tilapia feed obviously reduces the toxic action of biological toxin substances in the cotton seed meal and the rapeseed meal to the tilapia, obviously improves the meat quality of the tilapia, and is not added with any antibiotics, hormone and veterinary drug; the growth speed of the tilapia is fast, the feed coefficient is low, and the feed has good stability in water, and does not pollute aquaculture water bodies easily; and the materials are simple and easily obtained, the cost is low and the aquaculture cost can be greatly reduced.
Owner:ZHAOQING EXCELLENT BIOTECH
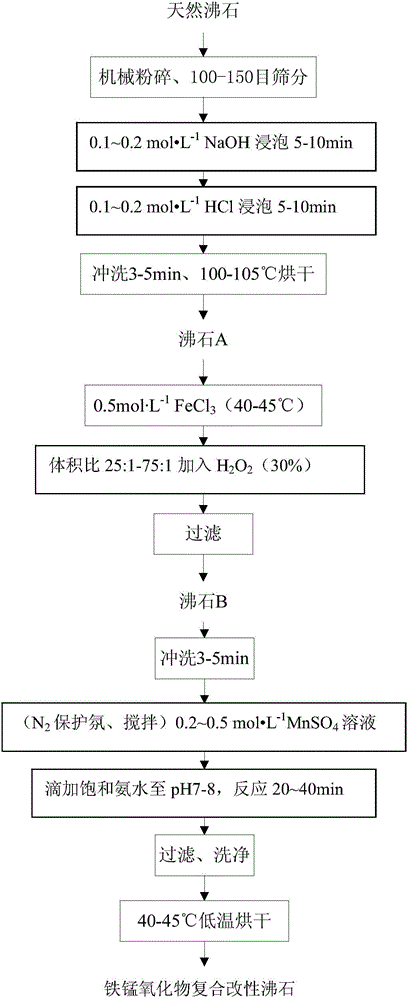
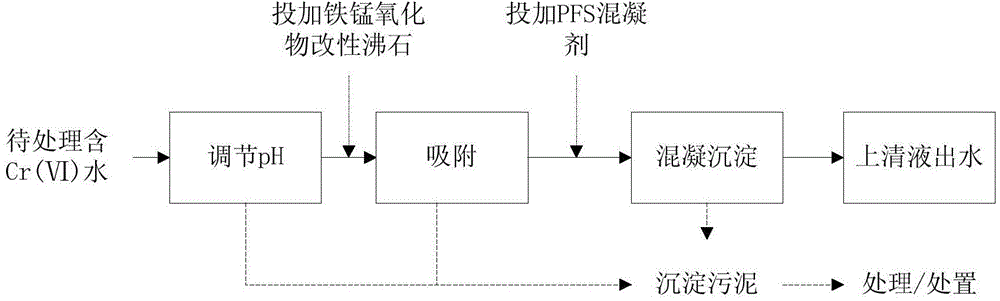
Iron and manganese oxide composite modified zeolite as well as preparation method and application thereof
ActiveCN104645932AIncrease surface areaAdaptableOther chemical processesAluminium silicatesWastewaterManganese oxide
The invention discloses iron and manganese oxide composite modified zeolite as well as a preparation method and application thereof. The preparation method particularly comprises the following steps: crushing and sieving natural zeolite, and carrying out acid and alkali pre-treatment; mixing the zeolite with an FeCl3 solution with a certain concentration to react to prepare an iron oxide modified zeolite material; mixing the iron oxide modified zeolite material with an MnSO4 solution with a certain concentration to react; and washing and drying at a low temperature to obtain an adsorption material which has a higher adsorption capability on Cr(VI) and a larger specific surface area. According to the iron and manganese oxide composite modified zeolite, the natural zeolite and the loaded iron oxide and manganese oxide are compounds in nature, and thus the iron and manganese oxide composite modified zeolite has no biological toxin, is safe to use and has no pollution. The method has the advantages that raw materials are easy to obtain, a preparation process is simple, reaction conditions are moderate, and the preparation cost is relatively low; and the method is particularly suitable for treating low-concentration industrial wastewater and sudden Cr(VI) pollution accident drinking water, and has a wide application prospect.
Owner:JINAN UNIVERSITY
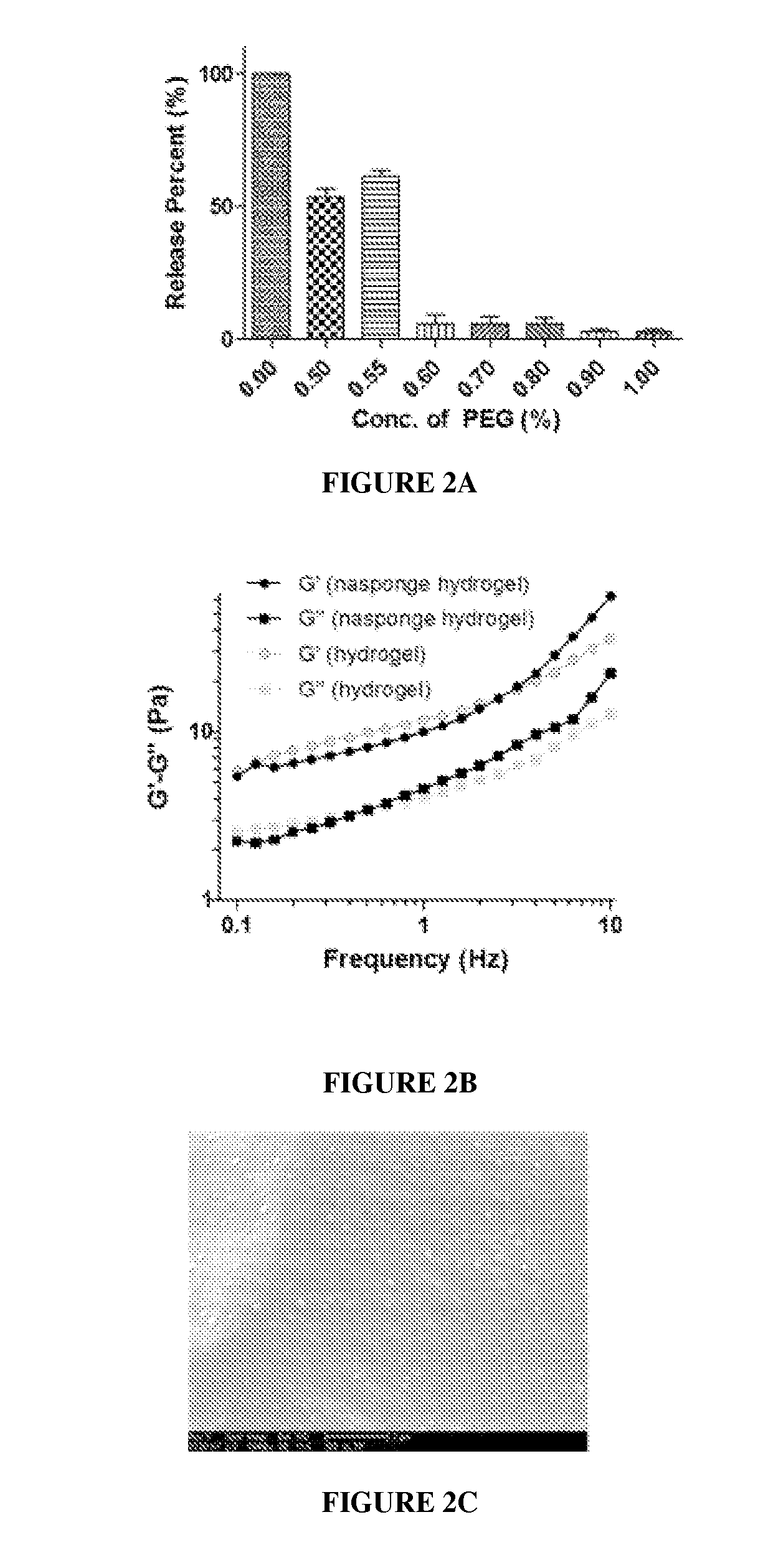
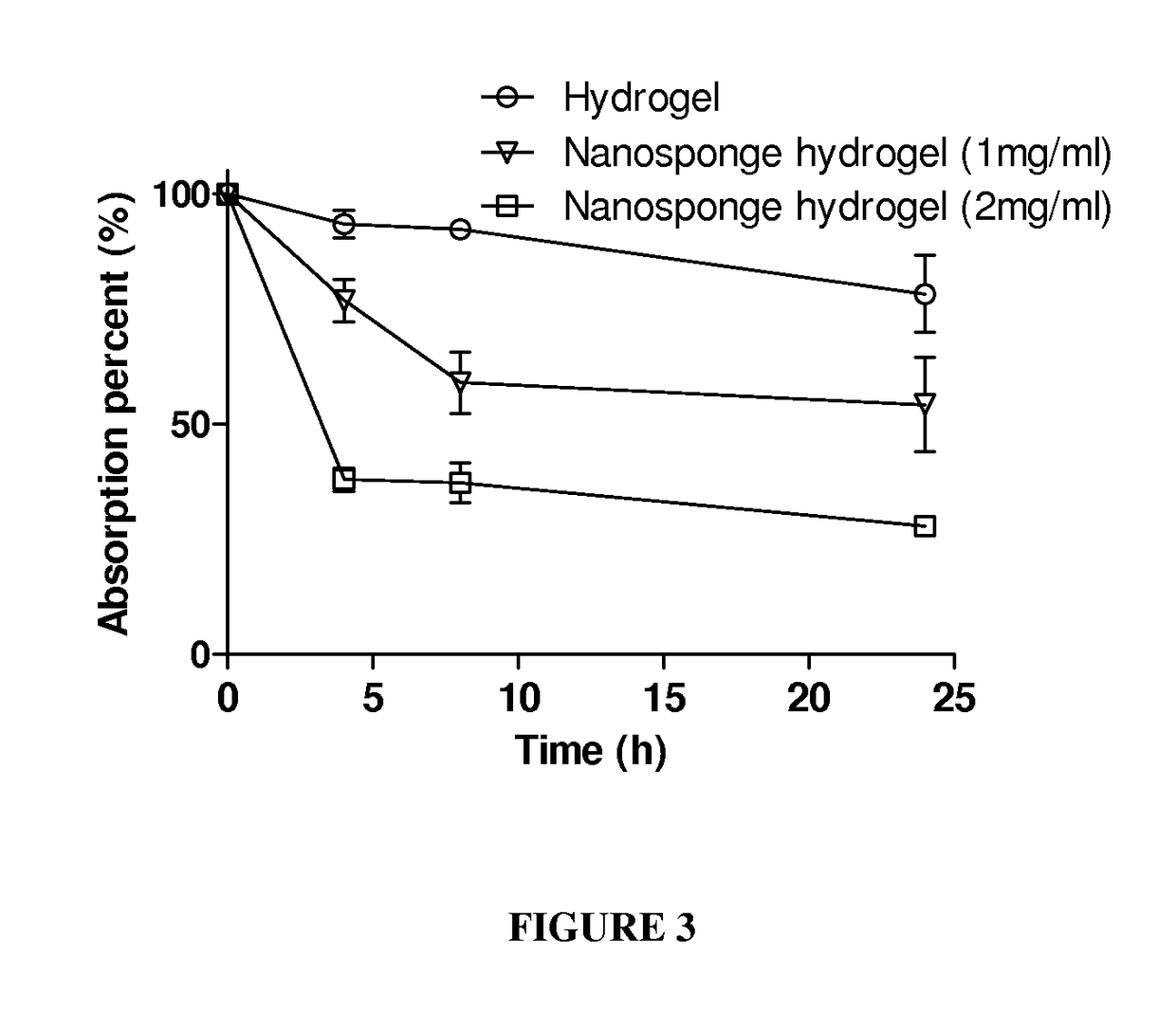
Hydrogel Toxin-Absorbing or Binding Nanoparticles
ActiveUS20170079909A1Reduce the impactImprove the bactericidal effectPeptide/protein ingredientsAerosol deliveryMicroorganismNanoparticle
The present invention provides for compositions comprising a polymeric hydrogel impregnated with a toxin-absorbing or binding nanoparticle. The present invention also provides for the use of the above compositions for decreasing or neutralizing the effect of a toxin, or for treating or preventing an infection by a microbe that produces a toxin, in a subject. The exemplary toxin is a biological toxin such as a viral, bacterial, fungal, plant or animal toxin.
Owner:RGT UNIV OF CALIFORNIA
Functional protective material, in particular for use in protective clothing
ActiveUS20140182050A1Avoid saturationHigh wearing comfortChemical protectionHeat protectionWater vaporMembrane surface
The invention relates to a functional protective material, in particular having a protective function with respect to chemical poisons and / or biological toxins and / or chemical and / or biological hazardous materials, such as warfare agents, wherein the functional protective material has a multi-layer structure that comprises a planar, in particular textile, substrate material and a membrane that is associated with the substrate material, in particular connected to the substrate material, wherein the membrane in itself is permeable to air and water vapor and therefore is provided with a plurality of micropores preferably distributed substantially evenly over the membrane surface. According to the invention, an operating parameter of the membrane can be changed in such a way that the membrane assumes two different operating states, wherein in the first operating state the micropores of the membrane are open and in the second operating state, the micropores of the membrane are at least predominantly closed.
Owner:BLUCHER GMBH
Method for preparing biotoxin
InactiveCN102675411AImprove insecticidal effectMicroorganism based processesPeptide preparation methodsCarboxyl radicalAbamectin
The invention provides a method for preparing biotoxin. The method comprises the following steps of: activating bacillus thuringiensis strains, performing fermentation culture, cracking, and centrifuging and purifying insecticidal protein of the bacillus thuringiensis; constructing a spacer arm containing carboxyl at abamectin C4''-OH by adopting a succinic anhydride method; and coupling the abamectin and the insecticidal protein of the bacillus thuringiensis under coaction of ethylene dichloride (EDC) and n-hydroxysuccinimide (NHS) which serve as cross linkers, performing ultra-filtration and centrifugal purification on the coupled product, and thus obtaining the biotoxin. The method has the advantages that the biotoxin with a good pest killing effect is obtained and has a good application prospect in prevention and control of agricultural pests.
Owner:福建省农业科学院农业生物资源研究所
Detection method for rapidly screening various pesticides and biotoxins in aquatic products
ActiveCN112379022AHigh sensitivitySimple methodComponent separationResolution (mass spectrometry)Mass Spectrometry-Mass Spectrometry
The invention discloses a detection method for rapidly screening various pesticides and biotoxins in an aquatic product. The detection method comprises the following steps: (1) preparing a standard solution; (2) carrying out QuEChERS extraction and purification of a sample; (3) carrying out concentration; and (4) carrying out analyzing by an ultra-high performance liquid chromatography high-resolution mass spectrometer. QuEChERS is used for pretreating the aquatic product, meanwhile, ultrahigh performance liquid chromatography quadrupole / electrostatic field orbitrap mass spectrometry (UPLC-Q Exactive Orbitrap HRMS is combined to conduct high-throughput measurement on 93 kinds of pesticide and 16 kinds of biotoxin residues in the aquatic product, and the method is sensitive, rapid, simple,accurate, time-saving, stable, high in practicability and capable of meeting the detection requirements of the current market.
Owner:张宪臣
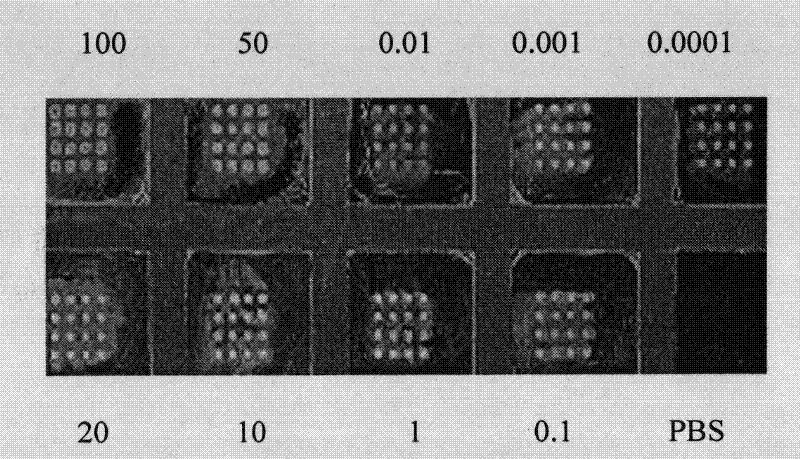
Method for quantitative detection of biological toxins
InactiveUS20070172904A1Overcome disadvantagesBioreactor/fermenter combinationsMaterial nanotechnologyFood industryImmobilized Antibodies
The invention relates to analytical chemistry and to quantitative immunochemical analysis, in particular, to a method for immunochemical quantitative detection of various biological toxins by using biological microchips. A biological microchip comprises an ordered array of three-dimensional hydrogel cells on a solid support, which are produced by a method of photo- or chemically induced polymerization and contain immobilized antibodies to various bacterial, plant and animal biotoxins, or biotoxins. The use of microchips makes it possible to analyze a sample simultaneously for the presence of several biotoxins. The proposed method for detecting biotoxins can be used in medicine, in food industry, and in environmental protection.
Owner:INST MOLEKULJARNOJ BIOLOGII IM V A EHNGELGARDTA RAN +1
Extracellular Matrix Materials as Vaccine Adjuvants for Diseases Associated with Infectious Pathogens or Toxins
InactiveUS20100136050A1Antibacterial agentsBacterial antigen ingredientsPrevention infectionCell-Extracellular Matrix
Disclosed are vaccines and vaccine adjuvants useful in the treatment and / or prevention of infection and diseases associated with infectious pathogens, such as tetanus, as well as diseases associated with biological toxins. Also provided are methods of preparing an adjuvant and the vaccine containing the adjuvant. Methods are also provided for vaccinating / immunizing an animal against infection and diseases associated with infectious pathogens, such as tetanus, and other diseases associated with biological toxins. Adjuvant materials are presented that are prepared from an extracellular matrix material. The adjuvants are demonstrated to enhance the immunogenicity of an infectious pathogen antigen or biological toxin antigen of interest, as well as to enhance the survival of an immunized animal.
Owner:UNIV OF NOTRE DAME DU LAC +1
Liquid-mass method for detecting various marine biotoxins in an aquatic product
ActiveCN110146632AReduce lossesFully purifiedComponent separationSpectrometerTandem mass spectrometry
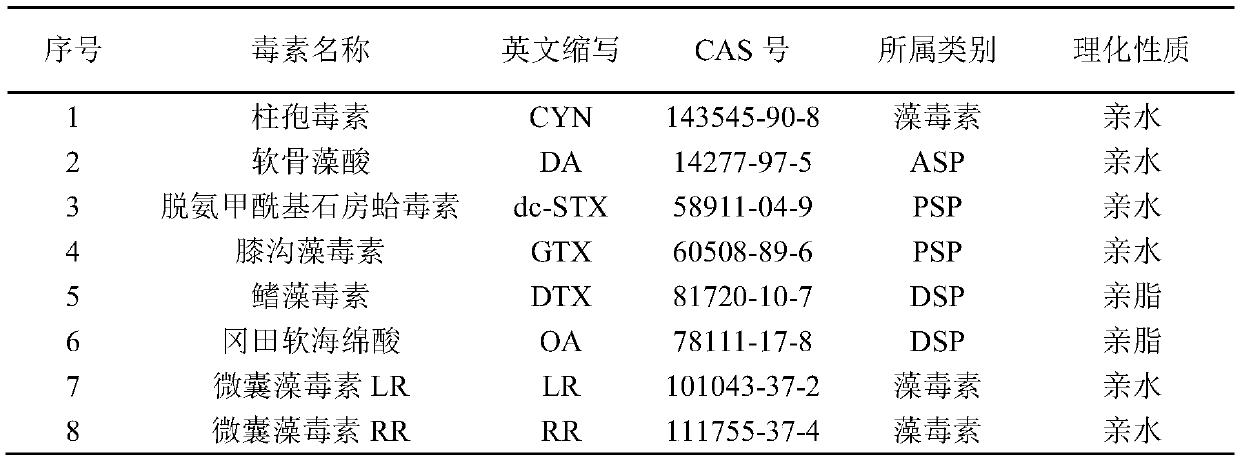
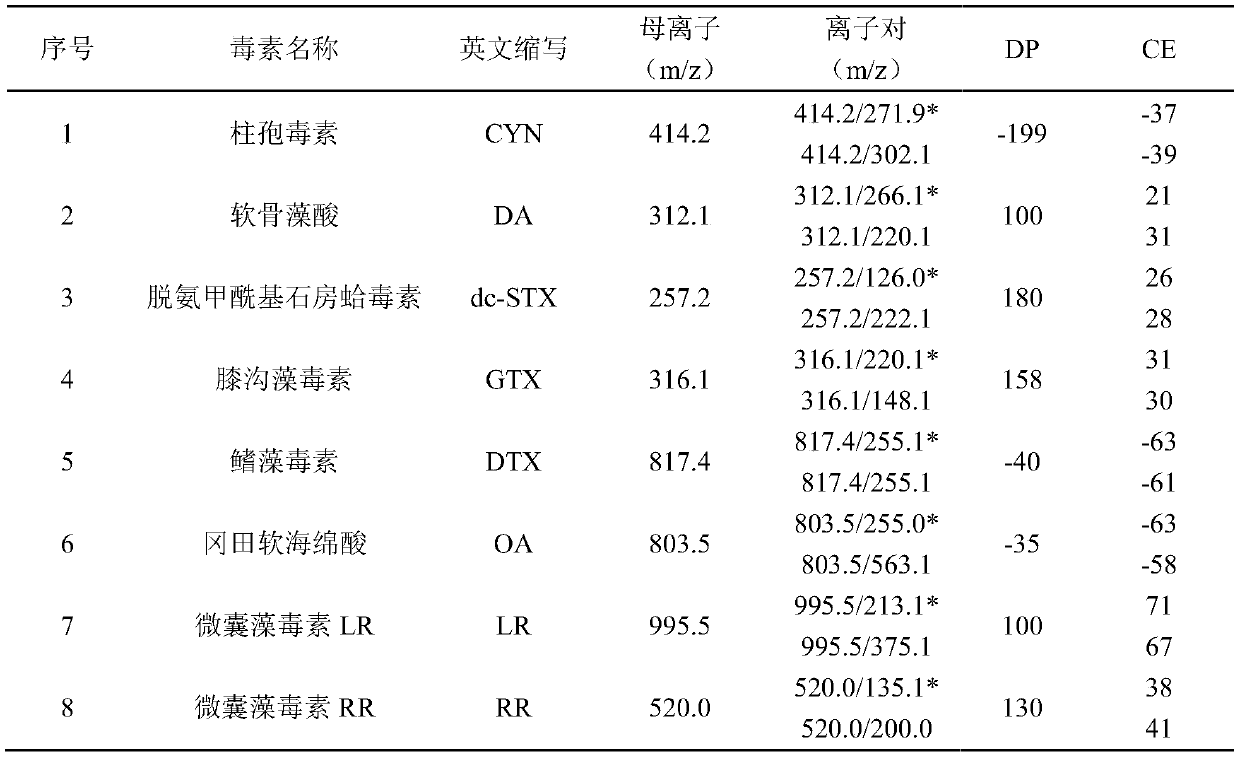
The invention discloses a liquid-mass method for detecting various marine biotoxins in an aquatic product. The sample of the aquatic product to be tested is extracted by using 0.1 vol% formic acid aqueous solution and acetonitrile in a classification way repeatedly, and layering is carried out to acquire upper organic phase and lower aqueous phase. The lower aqueous phase is added into an MCX-HLBseries column. Rinsing is carried out with a low proportion of methanol water. Elution is carried out with the organic phase purified by dSPE. Secondary elution is carried out with alkaline methanol.A high performance liquid chromatography-tandem mass spectrometer is used for detection after adding eluant with a dropper to bring the bottom of the meniscus to the line. An external standard methodis used for quantification to acquire the content of each component to be tested in the sample of the aquatic product to be tested. According to the invention, eight kinds of hydrophilic and lipophilic marine biotoxins can be detected at one time, and first-step liquid-mass high-throughput quantitative detection of hydrophilic and lipophilic marine biotoxins is realized.
Owner:NINGBO ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
Super-high-pressure ethylene compressor oil and preparation method thereof
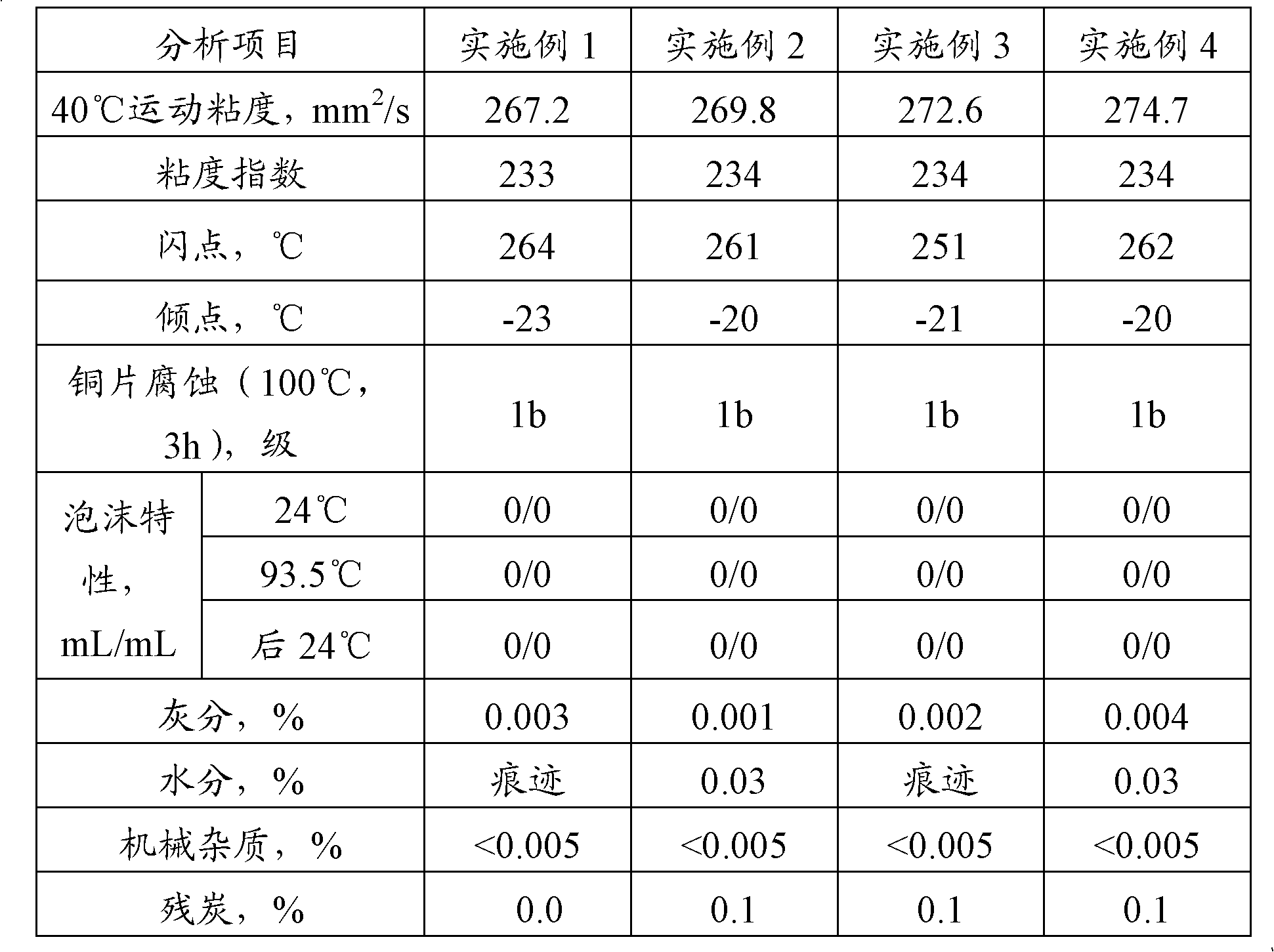
ActiveCN102311848AKeep healthyMeet the special requirements of oilLubricant compositionUltra high pressureAntioxidant
The invention provides super-high-pressure ethylene compressor oil, which contains the following components in percentage by weight: 93-99% of polyethers synthetic oil, 0.1-2% of antioxidant and 0-5% of anti-rusting agent. The super-high-pressure ethylene compressor oil provided by the invention takes the polyethers synthetic oil as the base oil and special additive compositions are added, so that the product can resist the dilution of ethylene gas and also can control the rise of viscosity under the extremely-high pressure to realize good lubrication; and the oil has good thermal oxidation stability and lubricity, low carbon deposition tendency and low biological toxin, so that the physical health of operators and safety and sanitation of mixing extremely little amount composition with polyethylene products can be guaranteed, and special requirements on the oil for a super-high-pressure compressor system can be satisfied.
Owner:CHINA PETROLEUM & CHEM CORP
Preparation method of citrinin immunoaffinity column
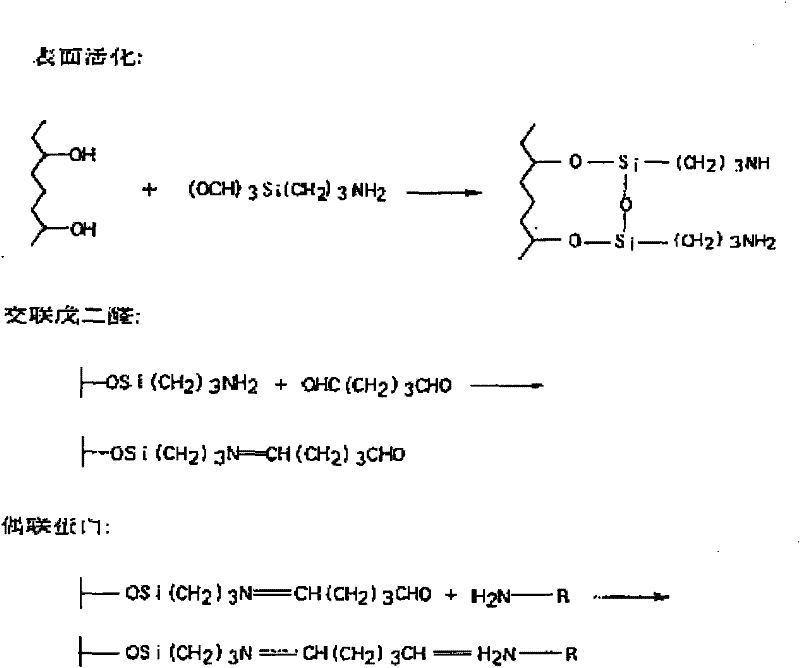
InactiveCN103157296AHigh purityIncrease concentrationSerum immunoglobulinsOther chemical processesCarrier proteinSolid phase extraction
The invention discloses a preparation method of a citrinin immunoaffinity column, belongs to the technical field of immunoaffinity chromatography and biotoxin detection, and discloses the preparation method of the citrinin immunoaffinity column. Immunogen is prepared by connecting citrinin and carrier protein through a formaldehyde condensation method. An immune animal gets an anti-citrinin antibody. Cupric embedded ionic polymer is prepared in a mass polymerization method. Immunoaffinity adsorbent which is prepared by adsorbing the polymer to the anti-citrinin antibody is filled into the citrinin immunoaffinity column which is prepared in a solid phase extraction pipe. The citrinin immunoaffinity column which is prepared in the preparation method of the citrinin immunoaffinity column is capable of combining with the citrinin in a specific mode, and cirtrinin solution with high purity and concentration is obtained through purification and enrichment. The maximum combination volume of the citrinin immunoaffinity column which is prepared in the preparation method of the citrinin immunoaffinity column is 597 ng, citrinin fortified recovery in a sample is 83.7% to 93.2%, and the recovery rate is no lower than 80% when the citrinin immunoaffinity column is repeatedly used for three times. The preparation method of the citrinin immunoaffinity column can be applied to rapid detection of the citrinin in fermented food.
Owner:SHANDONG NORMAL UNIV
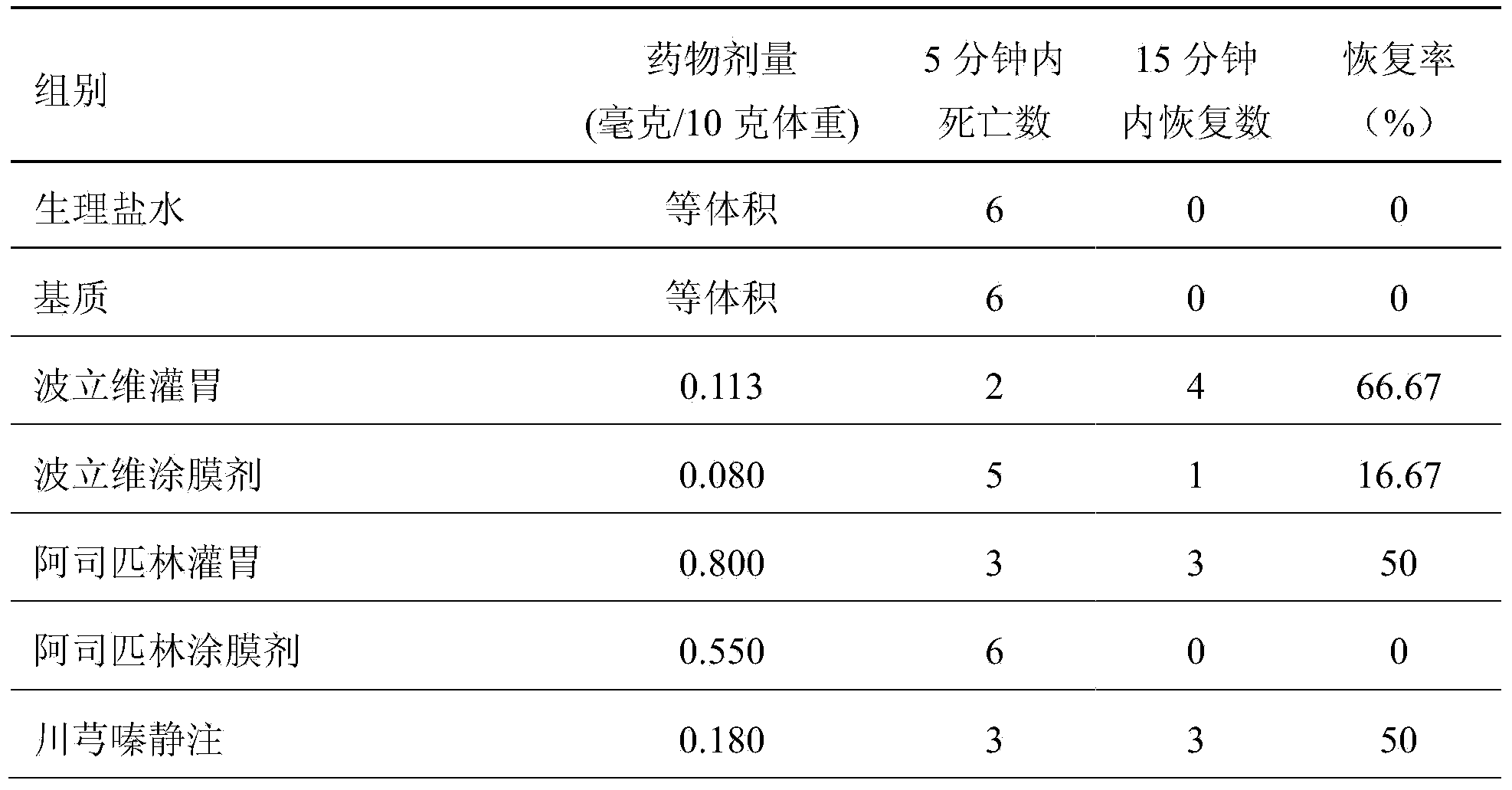
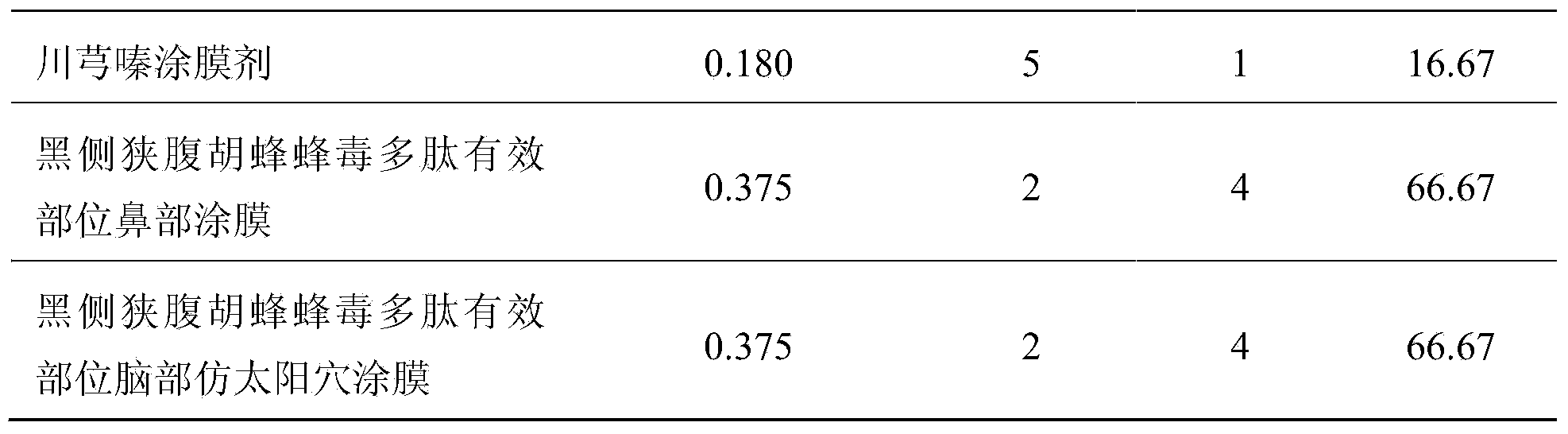
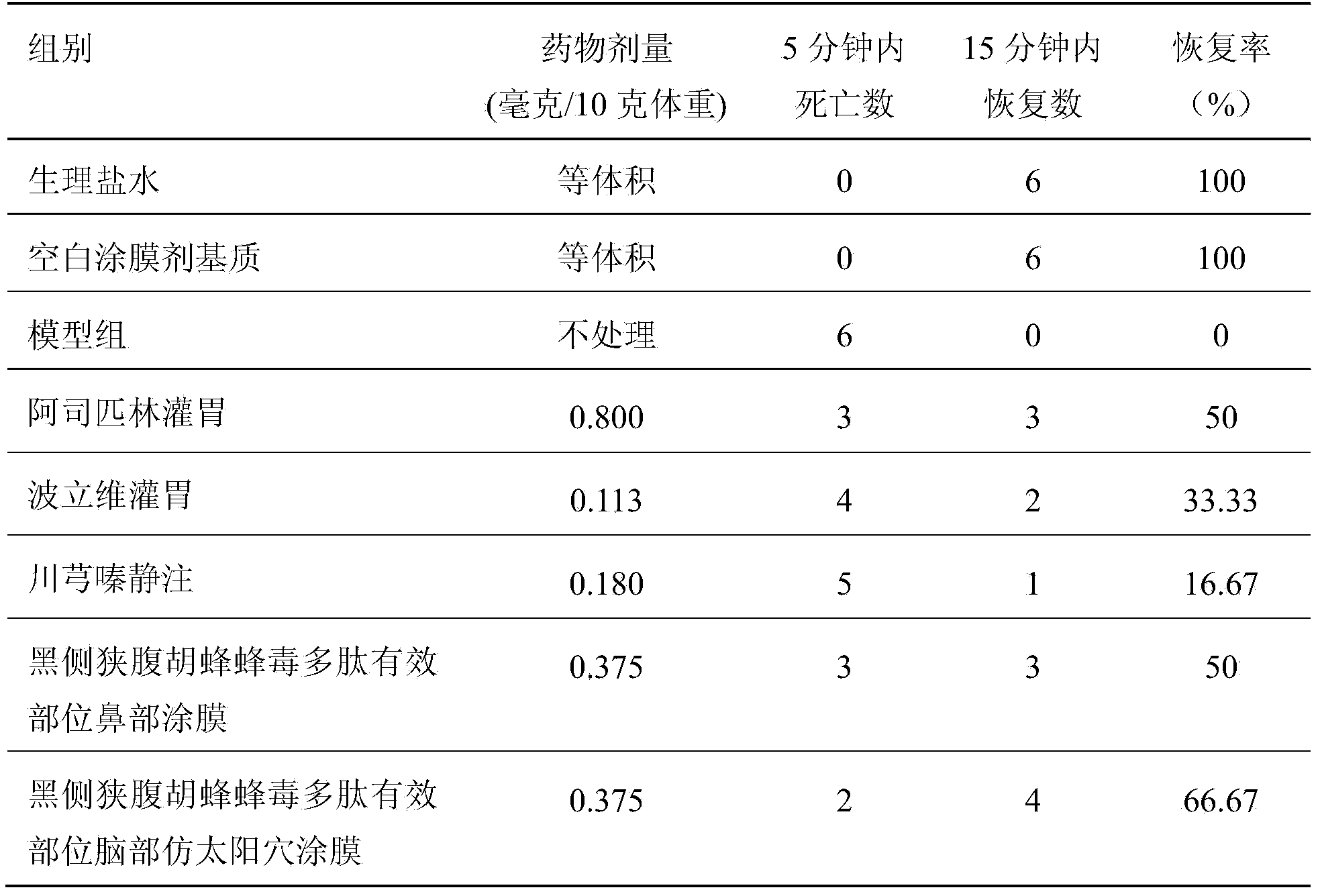
Preparation method of effective polypeptide components in Vespula insects, and medicinal uses of effective polypeptide components
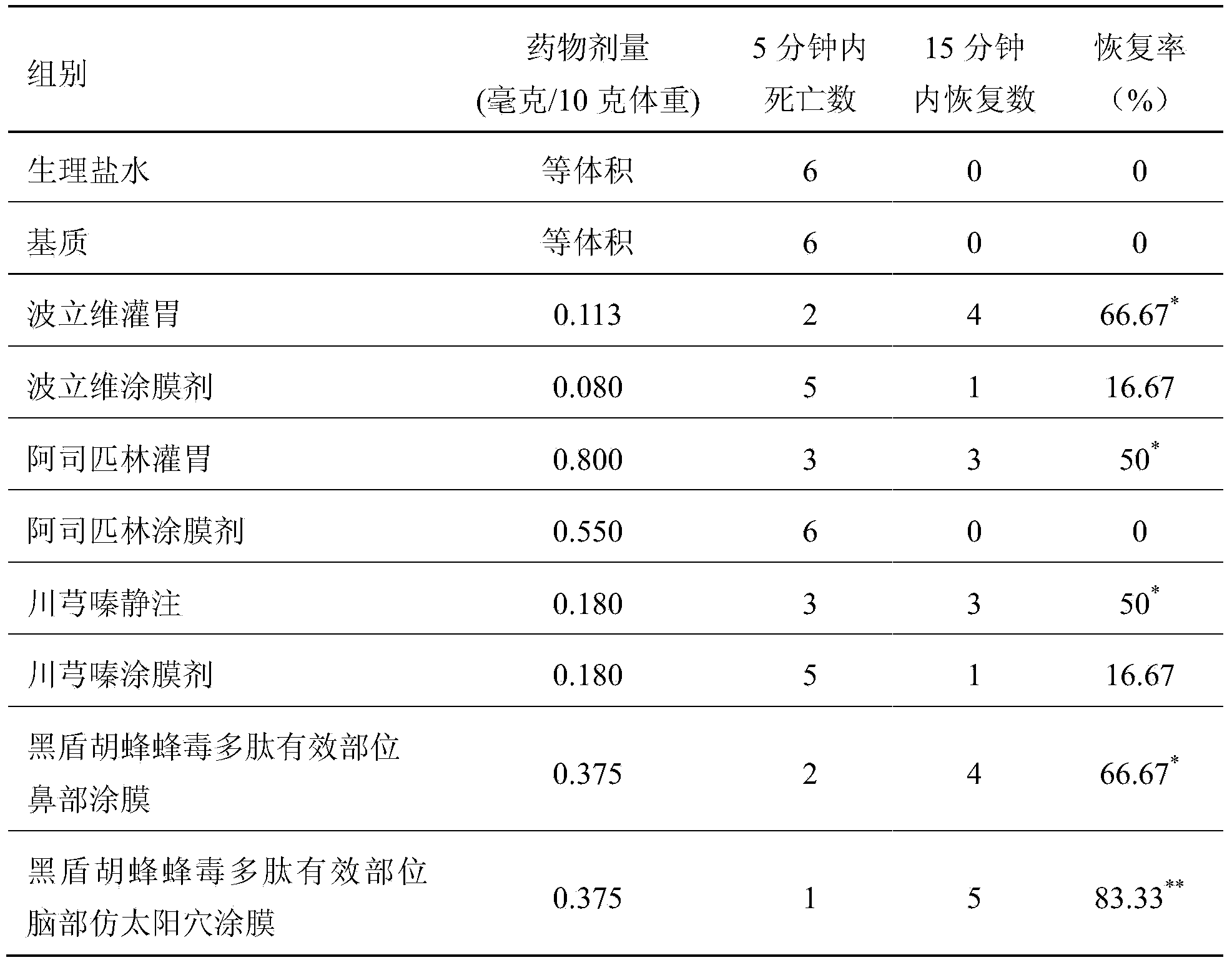
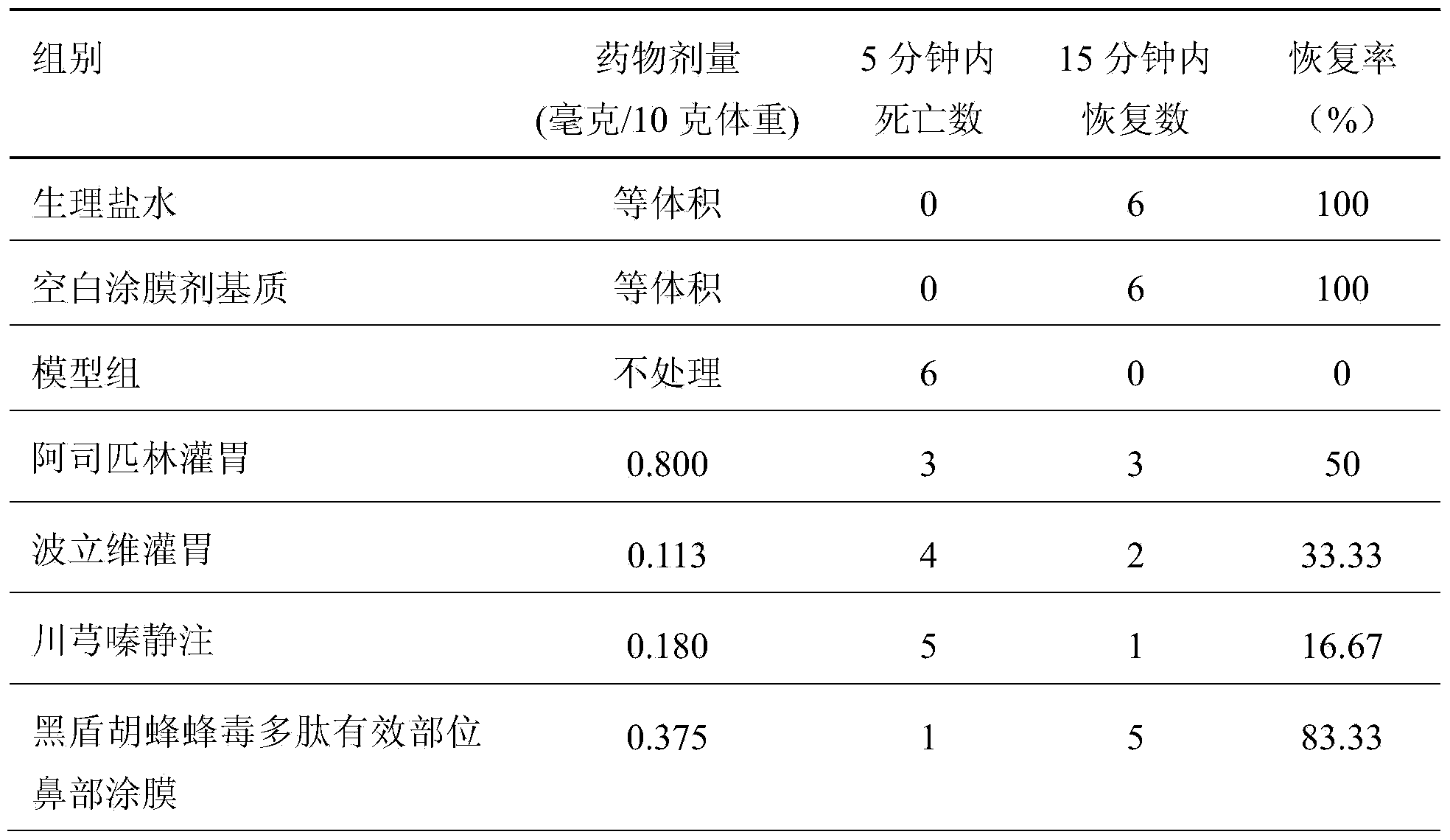
InactiveCN103665136AEasy to prepareLow costNervous disorderPeptide/protein ingredientsDiseaseDialysis membranes
The invention relates to a preparation method of effective polypeptide components in Vespula insects, and medicinal uses of the effective polypeptide components. The effective components are obtained through a step of dialyzing crude hornet venom through a 30KDa dialysis membrane, and a step of freeze-drying or reduced pressure concentration drying of the above obtained dialysis liquid. A series of pharmacodynamic experiments verify that the effective biotoxin polypeptide components and transdermal absorption preparations thereof can substantially control the cerebral thrombus, can recover the mobility of cerebral palsy animals, can mitigate the ischemic brain injury, have stronger pharmacodynamic performances than first-line medicines comprising plavix, Xingnaojing and the like, and can be expected to be used for preparing innovative biochemical medicines for preventing and treating cardiovascular and cerebrovascular diseases in order to prevent and treat ischemic cardiocardiovascular diseases, such as myocardial infarction, cardiovascular thrombus, cerebral thrombus, cerebral infarction, acute / chronic cerebral apoplexy, and sequelae caused by cerebral infarction injury, like lateral hemiplegia, hemidysesthesia, hemiopia, facial and lingual paralysis, aphasia and general paralysis.
Owner:DALI UNIV
Immunochip test method of staphylococcus enterotoxins and fumonisin
InactiveCN102455356AImprove stabilityReduce experiment costFluorescence/phosphorescenceImmobilized AntibodiesStaphylococcus
The invention relates to an immunochip test method of staphylococcus enterotoxins (SEA and SEB) and fumonisin (AFB1), so as to realize the simultaneous test on the same chip. According to the invention, for the test of AFB1, the monoclonal antibody of AFB1 is immobilized on the surface of a piece of aldehyde glass and then added to the chip together with a complete antigen labeled by a certain amount of Cy3, and the technical research on the immunochip can be carried out by using a competition method. For the test of SEA and SEB, a double-antibody sandwich method is adopted and the optimization of experiment conditions is carried out; in the test, SEA and SEB are added to a reaction tank, SE which is not combined is washed off after reaction, and then a corresponding labeled antibody is added so as to form an immobilized antibody- objected to be determinand-labeled antibody sandwich structure, and the intensity of a fluorescence signal is increased with increase of the concentration of the objected to be determinand. The method disclosed by the invention is simple in operation, low in cost, sensitive and rapid, the result can be stably and reliably obtained, and the whole test process needs 1-2 hours. A new method for rapid test of various biotoxins is provided.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Compositions and methods to inactivate and/or reduce production of microbial toxins
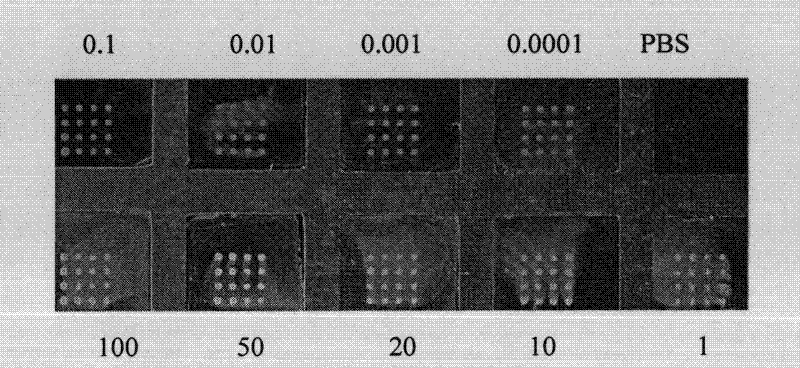
The present invention is related to compositions and methods to treat, ameliorate and / or prevent morbidity and / or mortality from microbial infections. In particular, bacterial infections that are associated with the production and release of bacterial toxins. For example, many Clostridia bacteria, such as Clostridium difficile, release toxins resulting in tissue and organ damage and death, even after antibiotic therapy that either reduces or eliminates the bacteria. In particular, various peptides, polypeptides, and proteins are disclosed herein that either inactivate Clostridium difficile toxin and / or reduce Clostridium difficile toxin production.
Owner:BIOLOG
Special antiseptic preservative agent and preservation method for marmoratus
InactiveCN104872272ALess chemical impuritiesSuppress generationMeat/fish preservation using chemicalsVitamin CBiological toxin
The invention discloses special antiseptic preservative agent and a preservation method for marmoratus. The special antiseptic preservative agent for the marmoratus includes 10 to 20 parts of natamycin, 15 to 150 parts of nisin, 10 to 50 parts of vitamin C, 20 to 40 parts of vitamin E, 10 to 30 parts of hyaluronic acid and 10 to 50 parts of ginkgol biloba extract by weight. According to the special antiseptic preservative agent and the preservation method for the marmoratus, the special antiseptic preservative agent composed of the nisin, natamycin and ginkgol biloba extract according to the proportion is capable of improving the ice preservation period of the marmoratus by more than 3 days. The special antiseptic preservative agent further lowers the residual biological toxin of the marmoratus.
Owner:ZHEJIANG OCEAN UNIV
Parischnogaster spp vespo bee venom polypeptide effective part and preparation and medical application thereof
InactiveCN103638060AProlong breathing timeSignificant antithromboticNervous disorderAnthropod material medical ingredientsDialysis membranesDisease
The invention relates to a Parischnogaster spp vespo bee venom polypeptide effective part and preparation and medical application thereof. Specifically, the effective part is prepared by dialyzing in the Parischnogaster spp rough bee venom with a dialysis membrane with molecular weight cutoff of 25KDa, and drying the eluate by means of freeze drying or concentration under reduced pressure. By a series of pharmacodynamic experiments, the inventor verifies that the biotoxin polypeptide effective part and transdermal absorption preparation thereof have the effects of remarkably preventing and treating generation of cerebral thrombus, recovering capacity for action of animal with cerebral palsy and relieving ischemic brain injury, have stronger pharmacodynamic performance than the first-line medicines, such as plavix and Xingnaojing, can be expectedly used for preparing cardiovascular and cerebrovascular disease prevention and treatment biochemical drugs for preventing and treating ischemic cardiovascular and cerebrovascular diseases, such as myocardial infarction, cardiovascular thrombosis, cerebral thrombosis, cerebral infarction and acute / chronic cerebral apoplexy, and sequelae caused by cerebral infarction injury, such as hemiplegia, hemidysesthesia, hemianopsia, facial-tongue paralysis, aphasia and general paralysis.
Owner:DALI UNIV
Aurea helianthus biological toxin expelling health-care oral liquid and preparation method therefor
InactiveCN105105142AReach bloodTo achieve the effect of meridiansYeast food ingredientsVinegar preparationEnzymatic hydrolysisDiuresis
The invention discloses an aurea helianthus biological toxin expelling health-care oral liquid and a preparation method therefor, and belongs to the biological enzyme preparation field. Aimed to toxins formed in the body in life, by utilization of an enzyme activity principle that biological enzymes generated during fermentation metabolism of aurea helianthus has specificity and high effectiveness inside and outside human body cells, toxins of each system of a human body are classified and degraded, and the enzymatic hydrolysis process is a process of changing toxins in the body into stasis scale. Then combined with sweating promoting, diuresis and laxative fermented food drugs, the stasis scale in the body is discharged from the body, and the purpose of body health care is achieved.
Owner:朱民生
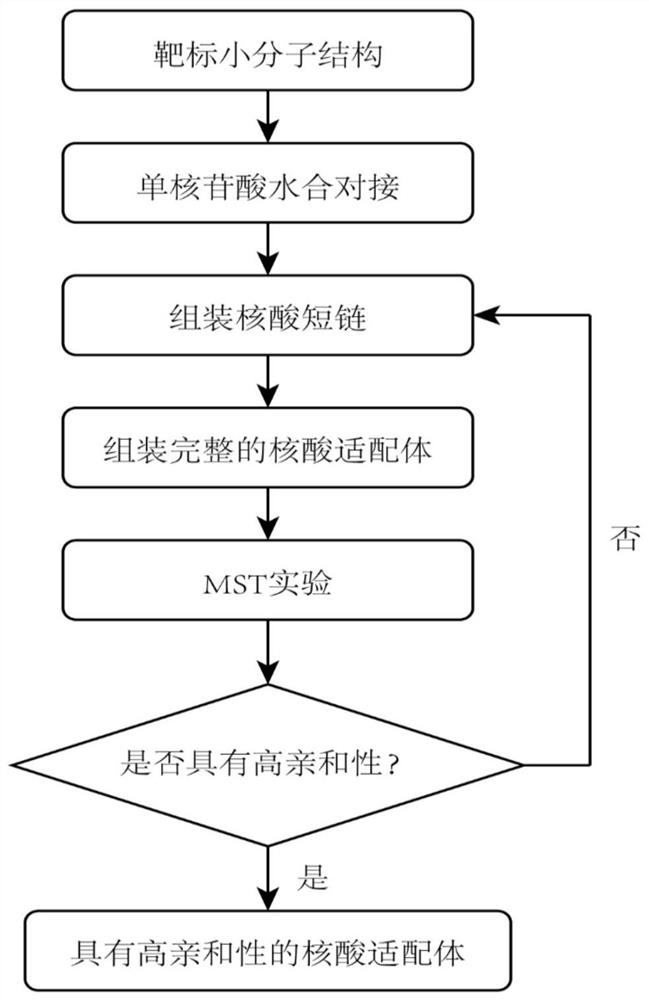
Aptamer design method based on single-nucleotide molecular docking
ActiveCN112210587AConformational stabilityThe experiment cycle is longMicrobiological testing/measurementDNA/RNA fragmentationAptamerNucleotide
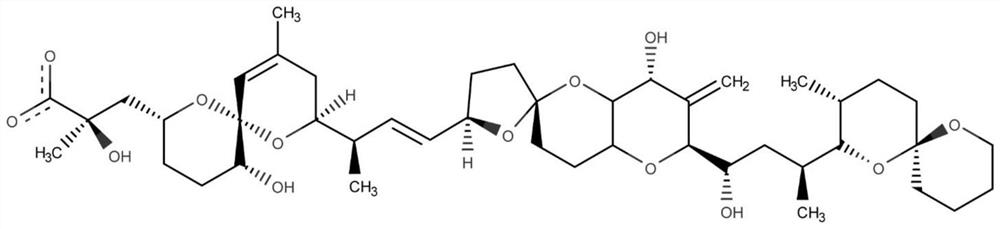
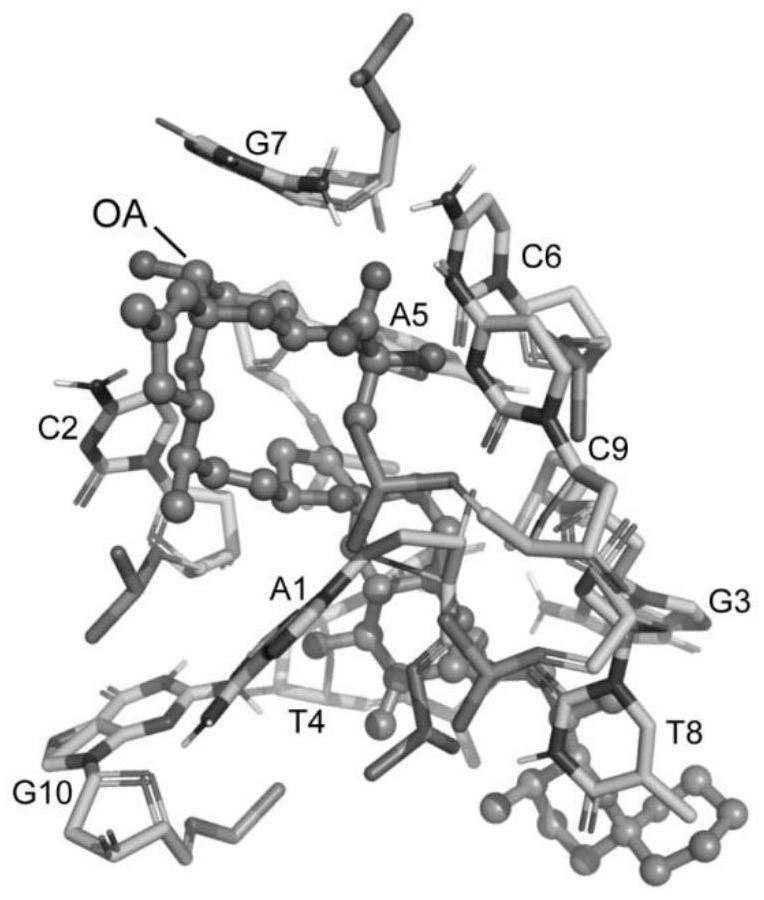
The invention belongs to the technical field of aptamer design, and particularly relates to an aptamer design method based on single-nucleotide molecular docking. Starting from an experimental structure of a target small molecule, positions of nucleotides combined around the target small molecule are obtained through hydration docking; then, the discrete nucleotides are assembled into a complete aptamer; and finally, the binding capacity of the designed aptamer and the target small molecule is detected by adopting an MST experiment. The aptamer design method in the invention has been used in the design of aptamers of toxin small molecule okadaic acid (OA); the toxin is a widely distributed marine biological toxin, and can cause diarrhea type shellfish poisoning, so that the detection of OAin marine products is of great significance to food safety; an aptamer OA-D1 targeting OA is obtained by design through a calculation method; the MST experiment verifies that the equilibrium dissociation constant of the aptamer and OA is 75.6 nM; and therefore, the calculation design method is reasonable and effective.
Owner:FUDAN UNIV
Air filtering absorber
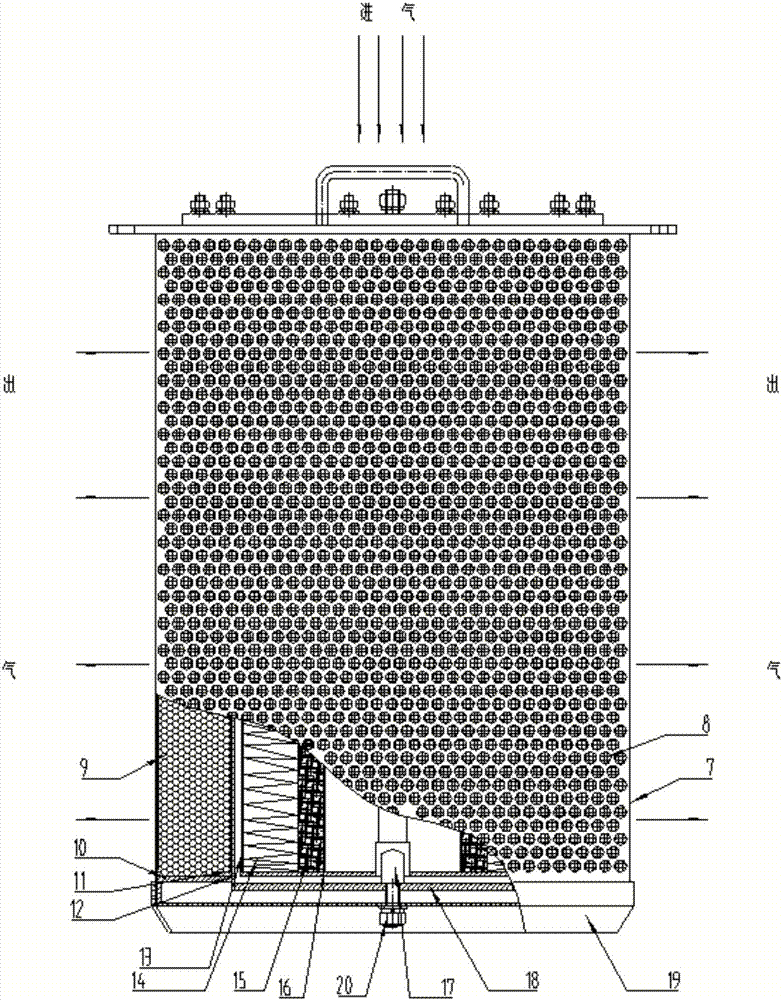
PendingCN107469487AReduce resistanceIncrease the adsorption areaDispersed particle filtrationTransportation and packagingActivated carbonAir filtration
The invention relates to an air filtering absorber. The air filtering absorber is of a cylindrical combined type structure, and is mainly composed of a filtering absorber housing, a paper filer, and an active carbon adsorption layer; the filtering absorber housing is composed of an internal pore plate, an external pore plate, an internal screen net, an external screen net, a pressing pad, and a sealing plate; the paper filter is composed of a primary efficiency filtering layer, a high efficiency filtering layer, and a paper filter internal pore plate. The air filtering absorber adopts the cylindrical combined type structure, resistance is low, adsorption area is large, multi-stage adsorption is realized, purifying capturing efficiency is high, high efficiency filtering material impregnated activated carbon is adopted, the air filtering absorber is invented based on pure physical principles, no energy consumption is caused, maintenance is simple and convenient. The air filtering absorber is suitable to be used in sealed spaces such as sealed defense works, vehicles, ships, and shelters, can be used for filtering radioactive ash, chemical toxins, and biological toxins, and purifying rate is increased to be 99.999% at a nominal air delivery.
Owner:718TH RES INST OF CHINA SHIPBUILDING INDAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com