Nucleic acid vaccine for preventing AIDS
A technology of nucleic acid sequence and nucleotide sequence, which is applied in the field of new vaccines to achieve the effects of reducing production costs, expanding production, and improving immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The selection and modification of embodiment 1 antigen
[0017] 1. Selection of the target gene
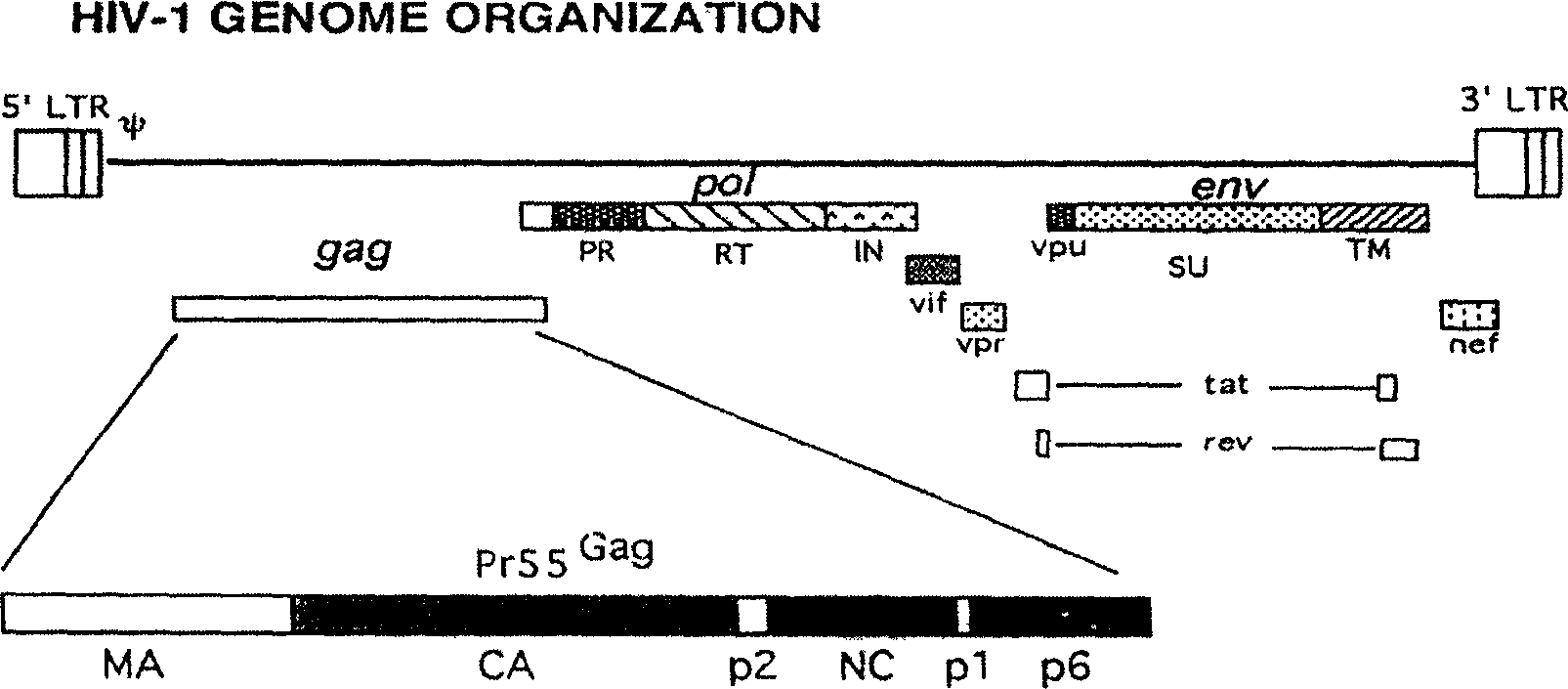
[0018] At present, the main HIV-1 epidemic strain in China is B / C recombinant HIV-1.
[0019] The gagpol gene sequence of the selected HIV-1 epidemic strain in China is shown as SEQ ID NO: 7
[0020] The env gene sequence of the selected HIV-1 epidemic strain in China is shown as SEQ ID NO: 8
[0021] The HIV-1 target gene we use to construct the AIDS vaccine here is based on the gene sequence of the above-mentioned B / C recombinant subtype, and then artificially synthesized the full sequence. The amino acid sequence expressed by the synthesized gene is the same as that of HIV-1 China The amino acid sequences expressed by the gagpol and env genes of the epidemic strains are consistent, but the gene expression efficiency is greatly improved.
[0022] With the above-mentioned gagpol and env genes as the genes of target antigens, their expression products constitute the most...
Embodiment 2
[0138] Example 2 Construction of Nucleic Acid Vaccine
[0139] 1. Selection of expression vector
[0140] 1.1 Overview.
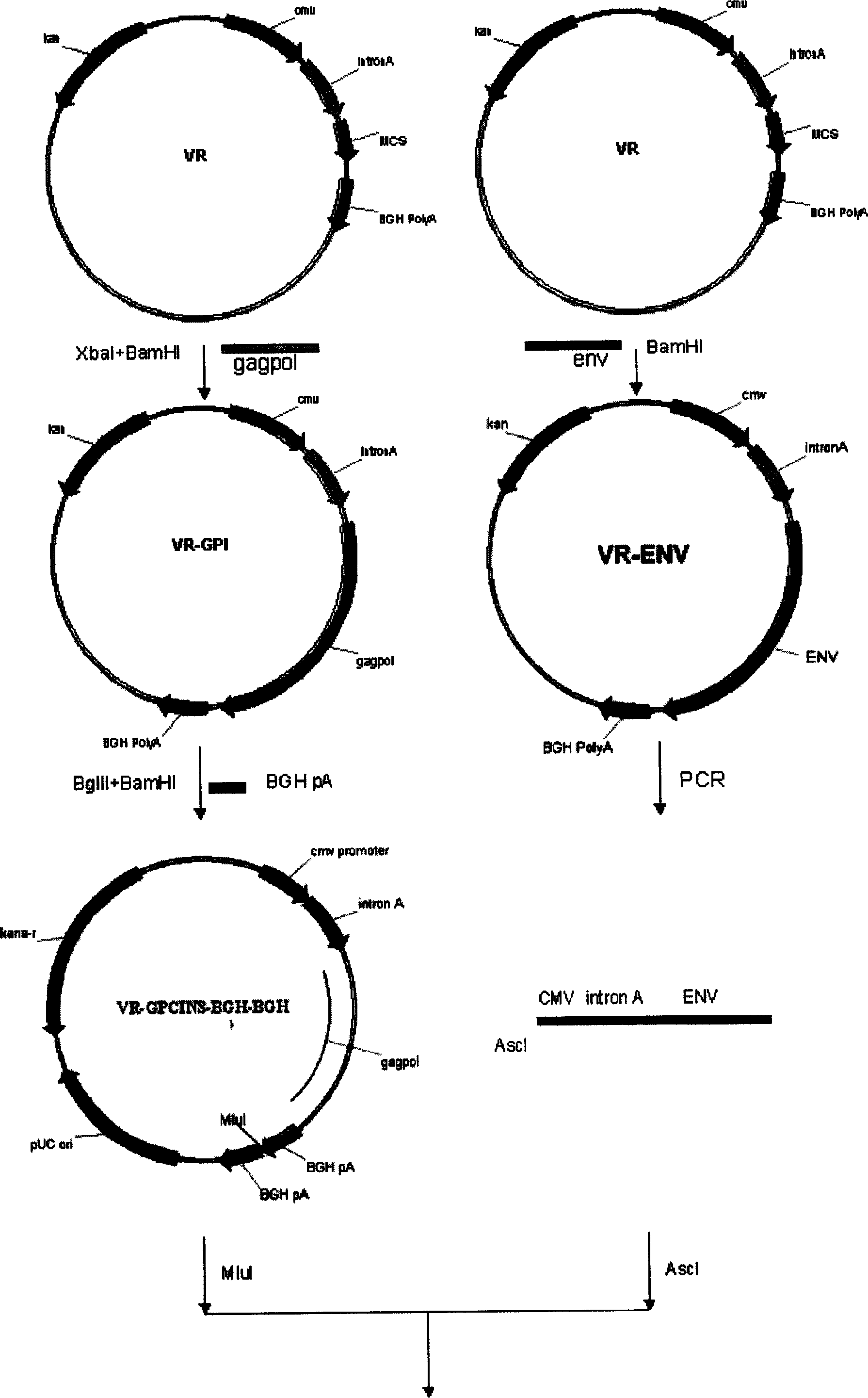
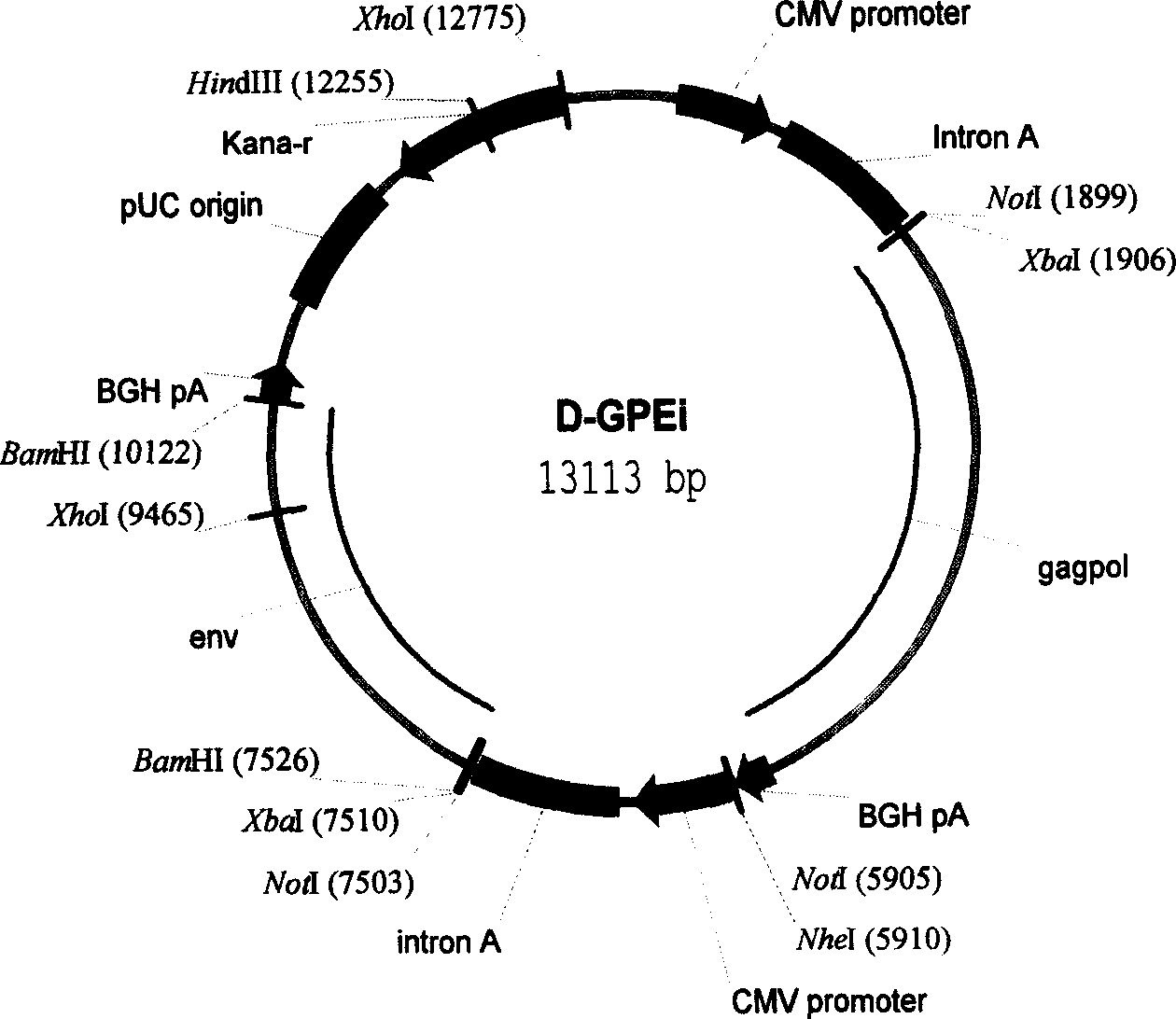
[0141] The construction of the vector (VR) of the DNA vaccine D-GPEi draws on the multiple cloning sites (including XbaI and BamHI, etc.) Factors, Intron A and BGH PolyA translation termination signal etc. conventional components. VR1012 is a carrier officially approved by the US FDA and can be used in clinical trials of human gene vaccines. The completed clinical trials have shown that its application in humans is safe.
[0142] The construction idea of this DNA vaccine is: use VR1012 as a template, copy the CMV promoter, IntronA and BGH polyA signal, and then clone the expression frame back into VR1012 by using the appropriate restriction site. Therefore, the new plasmid is composed of two CMV promoters, one kanamycin resistance gene, one prokaryotic cell high copy factor, two IntronA and two BGH polyA signals and other components. All parts of the ...
Embodiment 3
[0240] Example 3 Antigenicity Study of Plasmid D-GPEi
[0241] Objective: To investigate the antigenicity of plasmid D-GPEi through antigen expression of plasmid D-GPEi in mammalian cells.
[0242] The specific experimental methods and instrumental reagents are the same as those in Example 2 for the antigen expression of the 4 plasmid D-GPEi in mammalian cells.
[0243] Results and Discussion: In order to ensure the antigenicity of this AIDS vaccine is consistent with the antigenicity of HIV-1 epidemic strains in China (regional), we confirmed the gene sequence of HIV-1 epidemic strains in China according to the results of a large number of epidemiological studies. In order to increase the expression efficiency of the antigen in vivo, we modified the gene sequence of the wild-type HIV-1 and designed the gene sequence of the antigen part of the AIDS vaccine, but kept the amino acid residue sequence of the antigen protein unchanged.
[0244] In order to further confirm that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com