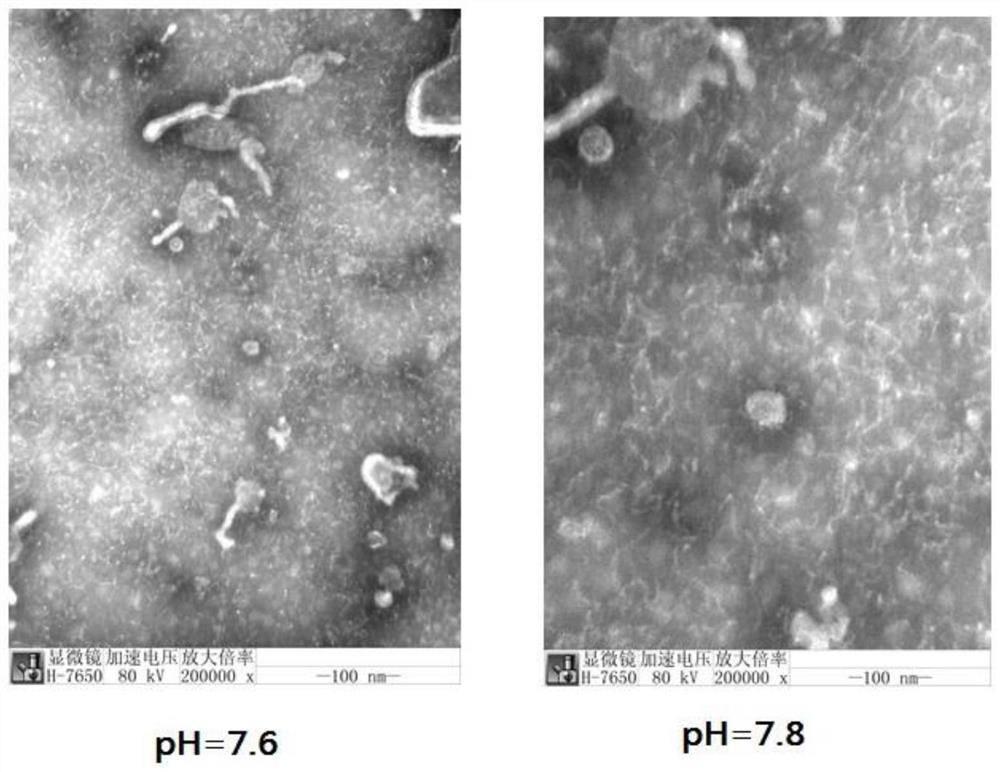
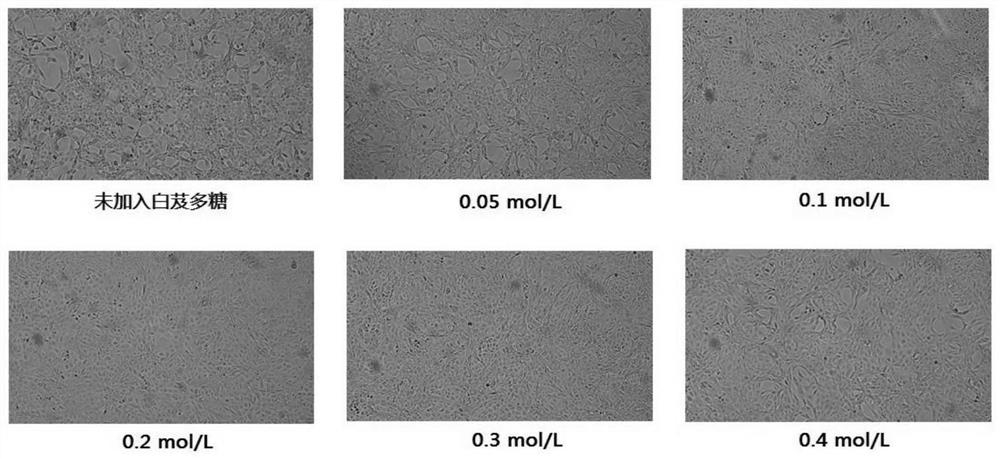
Method for preparing human Japanese encephalitis inactivated vaccine and vaccine
A Japanese encephalitis, inactivated vaccine technology, applied in microorganism-based methods, biochemical equipment and methods, artificial cell constructs, etc. Side effects, low safety and other problems, to achieve the effect of reducing pollution, improving vaccine safety, and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Serum-free adaptation of Vero cells
[0059] 1. Acclimatization of Vero cells
[0060] In this example, Vero cells derived from ATCC were acclimatized without serum using a mixed medium, and the specific operation steps are as follows:
[0061] (1) Recovery of Vero cells;
[0062] (2) domesticate Vero cells in the mixed culture medium;
[0063] Step 1: Culture the revived Vero cells in serum-containing medium. After the cells grow to a monolayer, wash the cells with DPBS, and then digest the cells with recombinant trypsin for passage;
[0064] Step 2: Culture the revived Vero cells in the first mixed solution. After the cells grow to a monolayer, wash the cells with DPBS, and then digest the cells with recombinant trypsin for passage;
[0065] Step 3: Culture the revived Vero cells in the second mixed solution. After the cells grow to a monolayer, wash the cells with DPBS, and then digest the cells with recombinant trypsin for passage;
[0066] Step 4: Cu...
Embodiment 2
[0083] Embodiment 2: the propagation of Japanese encephalitis virus in serum-free culture system
[0084] In the production of the Japanese encephalitis inactivated vaccine, the step of inoculating JEV on Vero cells to proliferate is included. In this example, a method for the propagation of Japanese encephalitis virus in a serum-free culture system is provided. In this method, JEV is inoculated onto Vero cells adapted to serum-free medium cultured in Example 1 , the specific operation steps are as follows:
[0085] Step 1, cell proliferation: culture the Vero cells acclimated in Example 1 in a serum-free medium, and discard the supernatant after the cells grow to a monolayer;
[0086] Step 2, virus inoculation: according to MOI=0.001-0.1, insert JEV and adsorb for 1 hour;
[0087] Step 3. Virus cultivation: add maintenance solution after virus inoculation, place at 33°C, 5% CO 2 Cultivate in an incubator;
[0088] Step 4. Virus harvest: change the medium after 1 day of cu...
Embodiment 3
[0095] Embodiment 3: Japanese encephalitis inactivated vaccine for humans
[0096] 1. Preparation of Inactivated Japanese Encephalitis Vaccine for Human
[0097] In this example, the Vero seed cells adapted to the serum-free medium obtained using the method described in Example 1 were added to the bioreactor, and the perfusion culture was carried out together with the microcarriers in the serum-free medium, and then the microcarriers were settled. Carriers and cells, replace the medium with maintenance solution, inoculate JEV to continue perfusion culture, collect the virus solution, and then concentrate, filter, inactivate, purify and freeze-dry to obtain the inactivated Japanese encephalitis vaccine for human use , the specific operation steps are as follows:
[0098]Step 1. Expansion culture of Vero cells: the Vero seed cells adapted to the serum-free medium obtained by acclimating using the method described in Example 1 were divided into 1.0×10 6 The inoculation density ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com