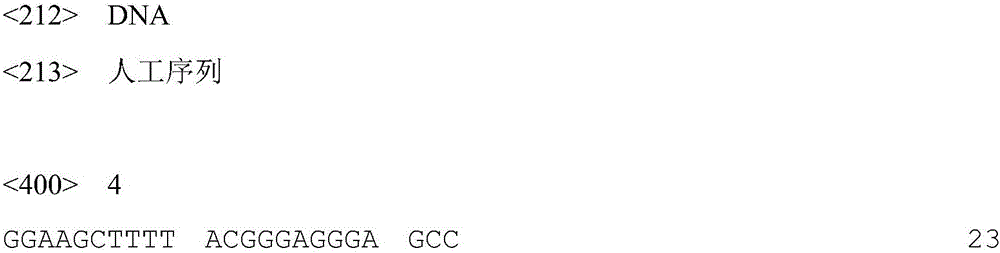I-group 4-type aviadenovirus genetic engineering subunit vaccine and preparation method thereof
A subunit vaccine and poultry adenovirus technology, applied in the field of vaccines, can solve the problems of lack of subunit vaccines, unreasonable selection of antigens, poor immune effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (fibrin C-terminal gene cloning and sequence analysis)
[0035] Design and synthesize a pair of primers:
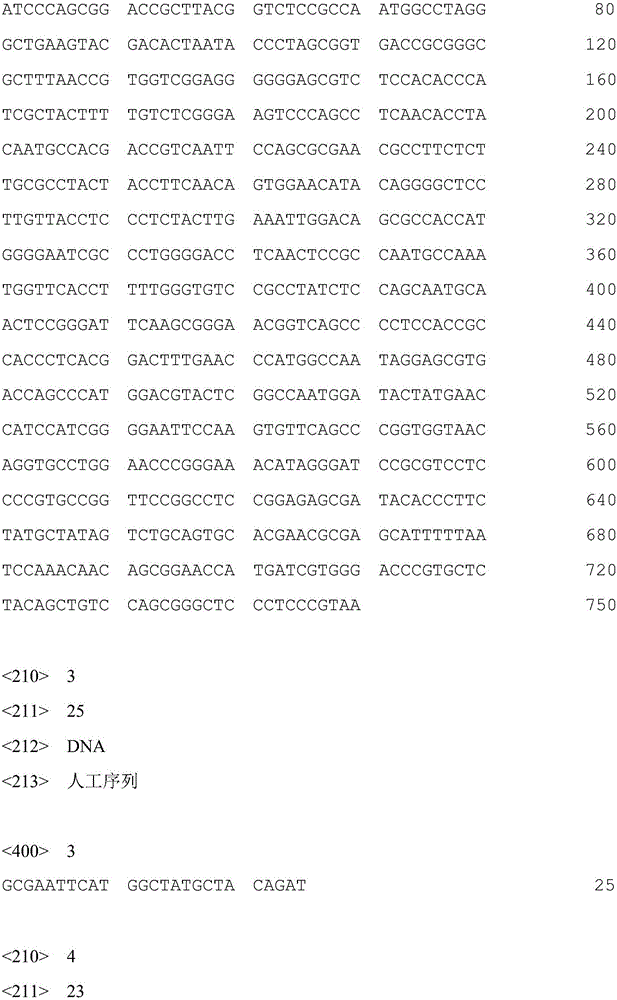
[0036] Primer 1: 5'-GCGAATTCATGGCTATGCTACAGAT-3';
[0037] Primer 2: 5'-GGAAGCTTTTACGGGAGGGAGCC-3'.
[0038] FAV-4 viruses were purchased from China Veterinary Drug Control Institute.
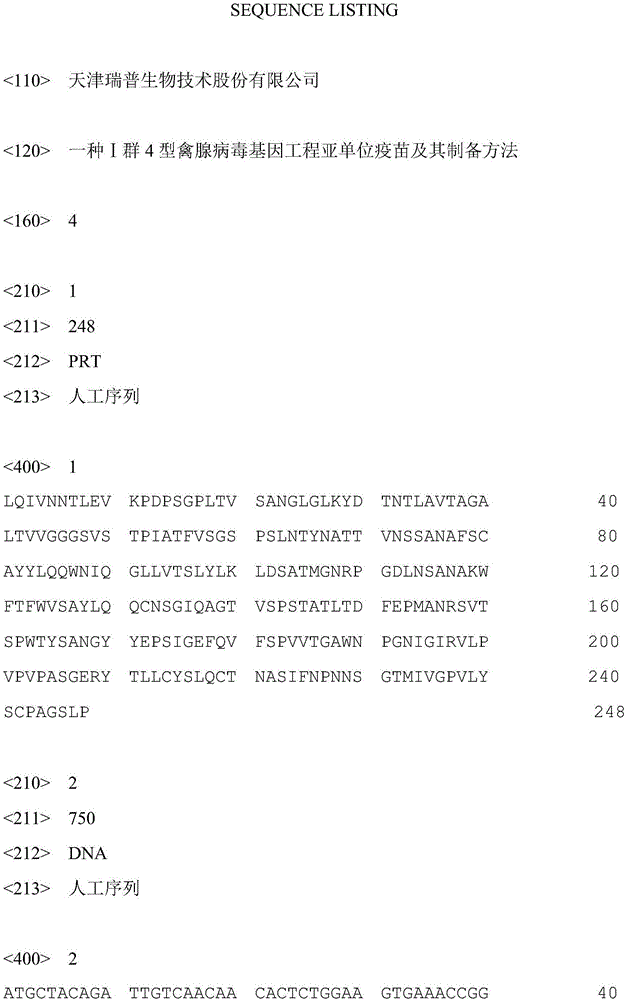
[0039] Using the FAV-4 virus as a template, denaturation at 94°C for 45s, annealing at 56°C for 45s, and extension at 72°C for 1min, a total of 30 cycles of PCR amplification were performed; the PCR product was directly cloned into the pMD-18T vector (TaKaRa Company) to construct pMD-18T-HHS; Sequencing was carried out by the dideoxy chain termination method, and the results showed that the gene encoding the fibrin C-terminus cloned from the FAV-4 genome had the nucleotide sequence shown in SEQ ID No: 2, which encoded The amino acid sequence is shown in SEQ ID No:1.
Embodiment 2
[0040] Embodiment 2 (construction of expression vector and engineering bacterium)
[0041] Digest pMD-18T-HHS and pET-32a with EcoR I and Hind III, perform 1% agarose gel electrophoresis on the digested products, recover the target fragment and pET-32a respectively, and then connect overnight at 16°C; Transformed into E.coliDH5α competent cells, picked a single colony and inoculated it into 2ml LB medium containing 100μg / mL ampicillin, cultured with shaking at 37°C for 12h, extracted the plasmid and identified it by double enzyme digestion, and obtained the expression plasmid pET-32a- HHS. Transform the recombinant plasmid into E.coliBL21(DE3), pick a single colony, inoculate into 200mL LB medium containing 100μg / mL ampicillin, culture with shaking at 37°C for 12h, then transfer to 4L of the same medium, shake at 37°C After culturing for 3 hours, IPTG was added to a final concentration of 0.5 mM, and culture was continued for 4 hours, followed by SDS-PAGE electrophoresis anal...
Embodiment 3
[0042] Example 3 (purification of the C-terminus of the fibrous protein of the recombinant group I group 4 avian adenovirus)
[0043] Pick a single colony of engineering bacteria in 200ml LB liquid medium (containing ampicillin at a concentration of 0.1g / L), and cultivate overnight at 37°C with constant temperature shaking; pour the overnight culture into a total of 4L of LB liquid at a volume ratio of 1:20 Culture medium (ampicillin concentration: 0.1 g / L) was cultured at 37°C with constant temperature and shaking for 3 hours; then IPTG was added to make the final concentration 5 mM, and cultured at 37°C with constant temperature and shaking for 4 hours.
[0044] Centrifuge the culture with a large-scale low-temperature refrigerated centrifuge, 8000rpm, 20min; discard the supernatant, add 100ml of binding buffer (20mmol / LTris.HCl, pH 8.0, 0.5mol / L NaCl, 5mmol / L imidazole) to suspend the cells, and then Perform 750W ultrasonic crushing (working time 10s, interval time 20s, cru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com