Nano antibody for resisting methicillin-resistant staphylococcus as well as preparation method and application of nano antibody
A nanobody, construct technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, antibodies, etc., can solve the consumption of essential lipoproteins and toxicity-related lipoproteins, bacterial cell death, mislocalization of unprocessed lipoproteins and other problems, to achieve the effect of easy separation and purification, not easy to aggregate, and good stability of structure and physicochemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
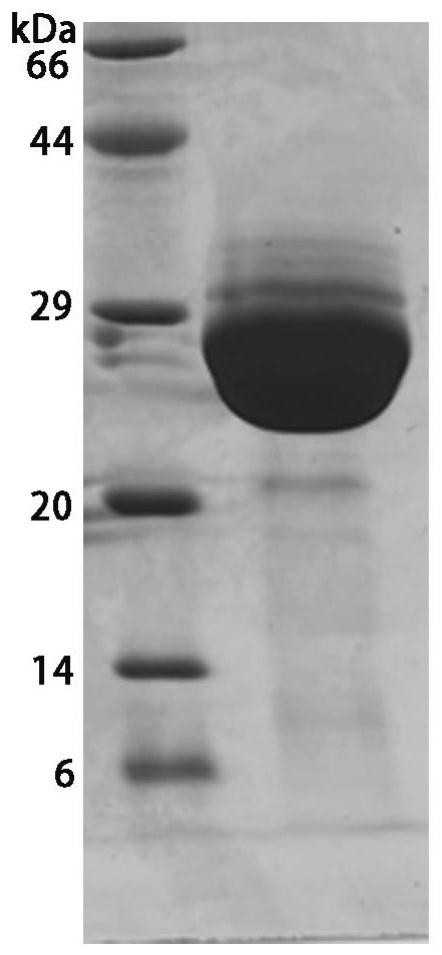
[0064] Expression and purification of embodiment 1.LspA-MRSA protein
[0065] LspA-MRSA (uniport: PODTC2, 330-541 amino acids) is constructed on the pET28a vector, and the reading frame coding sequence is respectively Honeybee Melittin secretion signal peptide (KFLVNVALVFMVVYISYIYAA) from N-terminal to C-terminal, Gly-Ser junction sequence, RBD object Protein sequence, Gly-Ser linker, 3C protease cleavage site (LEVLFQGP), Gly-Ser linker, Avi tag (GLNDIFEAQKIEWHE), Gly-Ser linker, and 10×His tag.
[0066] The expression of RBD was secreted and expressed in Trichoplusia ni High Five suspension cells. The supernatant after expression was collected, filtered through a 0.22um filter, added 20mM imidazole, mixed with 3mL Ni-Smart beads filler (SA035100), and incubated at 4°C Incubate for 3 hours under ambient agitation for binding. Then add the supernatant-resin mixture to the gravity column, collect the resin, wash with 10 column volumes of buffer A (150mM NaCl, 20mM Tris HCl pH 8...
Embodiment 2
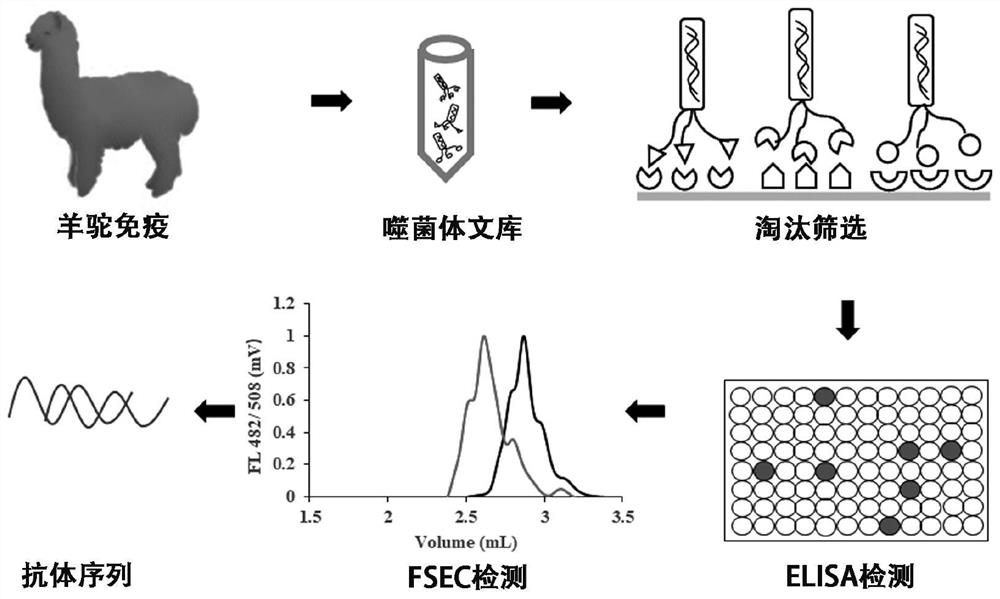
[0069] Example 2. Nanobody Screening
[0070] 2.1 Alpaca immunity
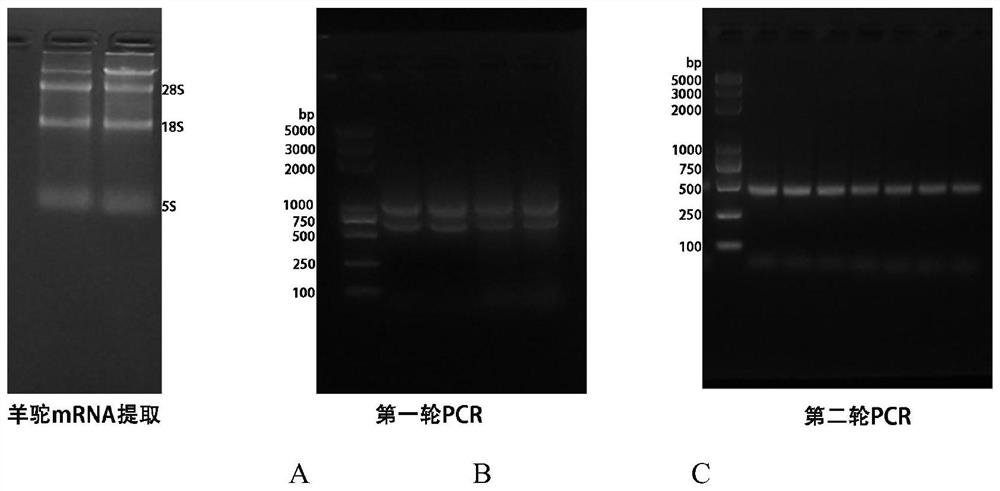
[0071] Before immunization, mix the immune antigen (2mg / mL, 500uL) prepared in Example 1 with the GERBU adjuvant (GERBUAJUVANT P#3111) at a volume ratio of 1:1 to form an emulsion, and then apply it to the neck of the alpaca The antigen-adjuvant emulsion was injected subcutaneously at 10 points close to the lymph nodes of the arch, and a booster immunization was carried out every 2 weeks. A total of 4 immunization injection experiments were carried out. Before the first immunization, 3 mL of blood was collected and coagulated at room temperature for 2 hours. Centrifuge at 3000 g for 5 min at room temperature, and collect the supernatant as pre-immune serum; the sera after the 1st to 4th immunizations were also collected according to this method, and then the serum antibody titers were determined by ELISA. After the 4th immunization, the titer of serum antibody is higher than 10 6 , collect 80 ml of blood in...
Embodiment 3
[0097] Example 3 Nanobody Purification
[0098] The gene encoding the nanobody was constructed into the pSb-init vector, and its C-terminus also had Myc tags and 6xHis tags. The constructed plasmid was transformed into Escherichia coli MC1061 for expression. The general process is as follows: the cells are treated with TB medium (0.17M KH) containing 25mg / L chloramphenicol 2 PO 4 and 0.72M K 2 HPO 4, 1.2% (w / v) peptone, 2.4% (w / v) yeast extract (yeast extract), 0.5% (v / v) glycerol (glycerol)), 37°C, 220rpm culture. When the bacteria grew to an OD of about 0.5, the temperature was lowered to 22° C., and the cells were continued to be cultured. When the OD reached about 1.5, 0.02% (w / v) arabinose was added to induce expression for 16 hours. Collect the cultured cells by centrifugation, resuspend each liter of cells with 20 mL of TES-high Buffer (0.5M sucrose, 0.5mM EDTA, and 0.2M Tris Tris-HCl pH 8.0), and incubate at 4°C for 30min. Then 40 mL of ice water was added and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com