Immunity-protective Acinetobacter baumannii surface antigen SurAl
A technology of Acinetobacter baumannii and protective antigen is applied in the identification field of Acinetobacter baumannii immunoprotective antigen, which can solve the problems of unclear biological function of protein and achieve the effect of effective immune protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the preparation of bacterial protein sample
[0037] Protein sample preparation
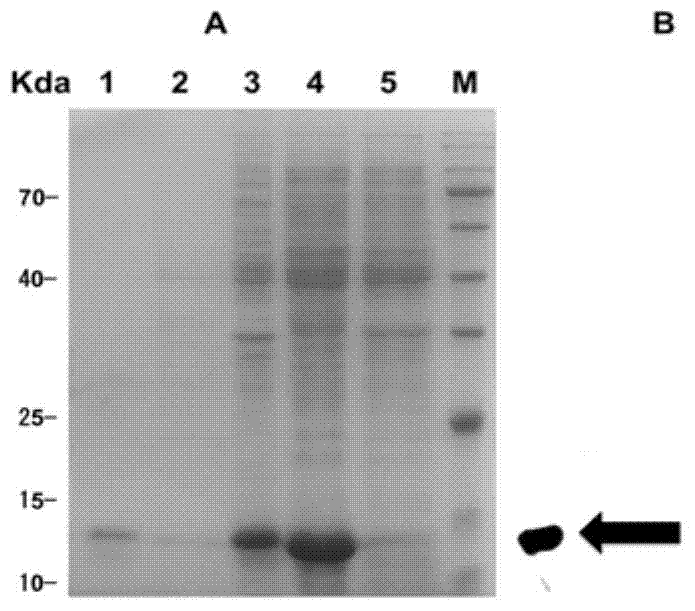
[0038] Take 1 mL each of Acinetobacter baumannii standard strain ATCC19606 and isolated strain CCGGD201101 frozen in a -80°C refrigerator, inoculate them in 200 mL of liquid LB medium and culture them at a constant temperature of 37°C and 180r / min for 7 hours. Weigh and mark the weight of 50mL centrifuge tubes before centrifugation, centrifuge at 10000r / min at 4°C for 10min, discard the supernatant, and wash twice with sterilized water, collect the bacteria and weigh the total weight, and calculate the weight of the collected bacteria . Add 10mL 0.1mol / L Tris-Cl to both ATCC19606 and CCGGD201101 to suspend the precipitate, then add 100uL protease inhibitor and 100uL ribozyme mixture and mix well. Then ultrasonic crushing was carried out at a power of 200w, 5s / time, with an interval of 15s between each time, and ultrasonication for 10min each. The whole process was operated ...
Embodiment 2
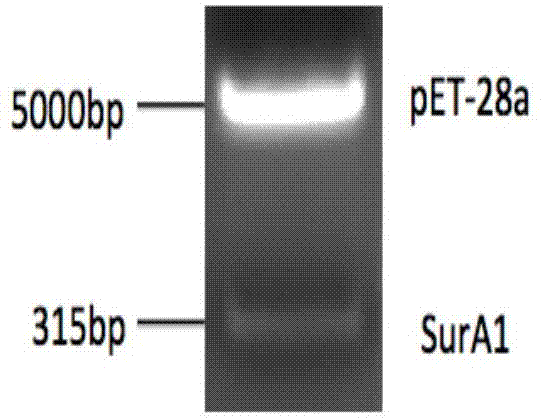
[0054] Embodiment 2, the expression of SurA1 protein
[0055] 1. Extraction of A.baumannii total DNA
[0056] Centrifuge 2ml of the overnight culture of A.baumannii CCGGD201101 at 12,000rpm for 1min, discard the supernatant, and extract it using the Total Bacterial Genomic DNA Extraction Kit (Cat. No.: Soleborn D1600-100).
[0057] 2. Preparation of SurA1 gene
[0058] According to the nucleotide sequence of the SurA1 gene, the primers were designed as follows:
[0059] Upstream primer: atggtaaaagattggattcccatc
[0060] Downstream primer: ctagtcttcaatgacgtgtaaacca
[0061] Using the total genomic DNA of A. baumannii as a template, the designed primers were used for PCR amplification.
[0062] Amplification system:
[0063] Ex taq DNA polymerase 1 μL
[0064] 10x Ex Taq DNA Polymerase Buffer 5 μL
[0065] dNTP Mix 0.4μL
[0066] Genome template 1 μL
[0067] Upstream primer 1 μL
[0068] Downstream primer 1 μL
[0069] wxya 2 O 40.6μL
[0070] Total 50μL
[0071] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com