Duck adenovirus 2 and DPV (Muscovy duck parvovirus) disease combined inactivated vaccine
A Muscovy duck parvovirus and dual vaccine technology, which is applied in the direction of viruses, vaccines, antiviral agents, etc., can solve the problems of poor immune effect, stress response of Muscovy duck flocks, virus mutation, etc., and achieve good commercial development Promising, effective immune protection, good safety profile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Isolation and Identification of Virus Strains
[0017] 1.1 Isolation and identification of GD strain
[0018] 1.1.1 Virus isolation of GD strain In 2015, the inventor successfully isolated a duck adenovirus type 2 from a group of muscovy ducks in a duck farm in Guangdong. Take the liver, spleen, kidney and other tissues of the diseased Muscovy duck, grind them, homogenize them with sterile PBS, freeze and thaw 3 times, and then centrifuge at 6000r / min for 10 minutes. Take the supernatant and add penicillin and streptomycin at 4°C overnight. After passing the filter sterility test, save it for future use. Muscovy duck embryo fibroblasts were inoculated with 1% virus filtrate, cultured in serum-free M199 culture medium, left standing at 37°C for 60-78 hours, obvious cytopathic changes could occur, and the virus fluid was collected.
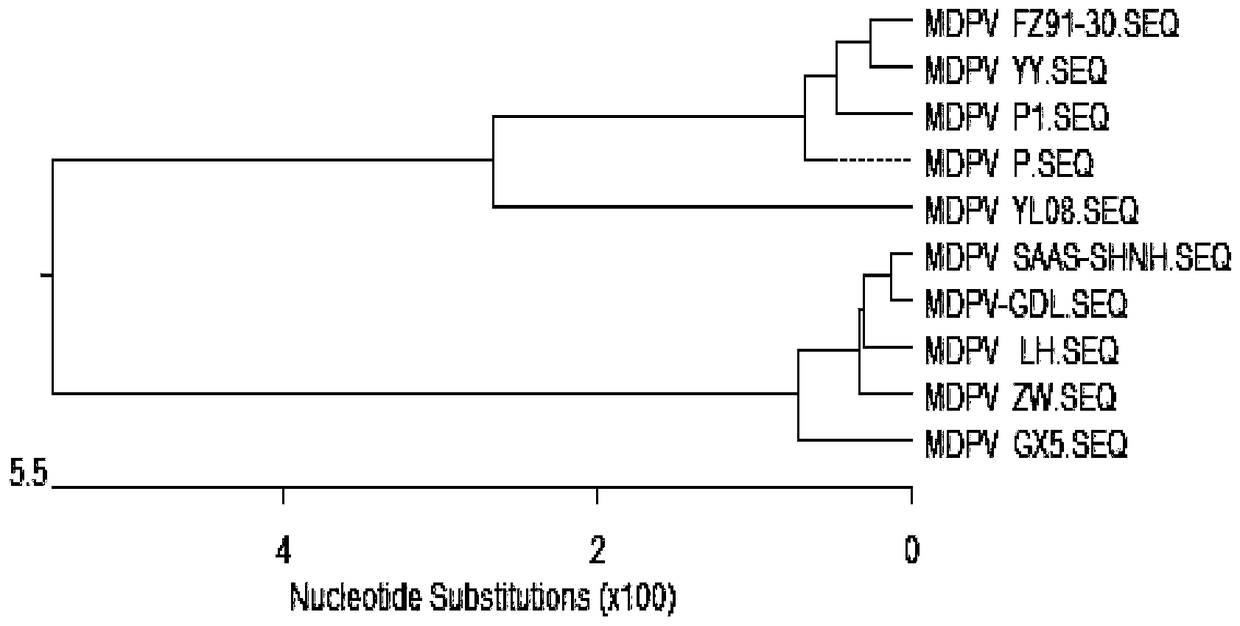
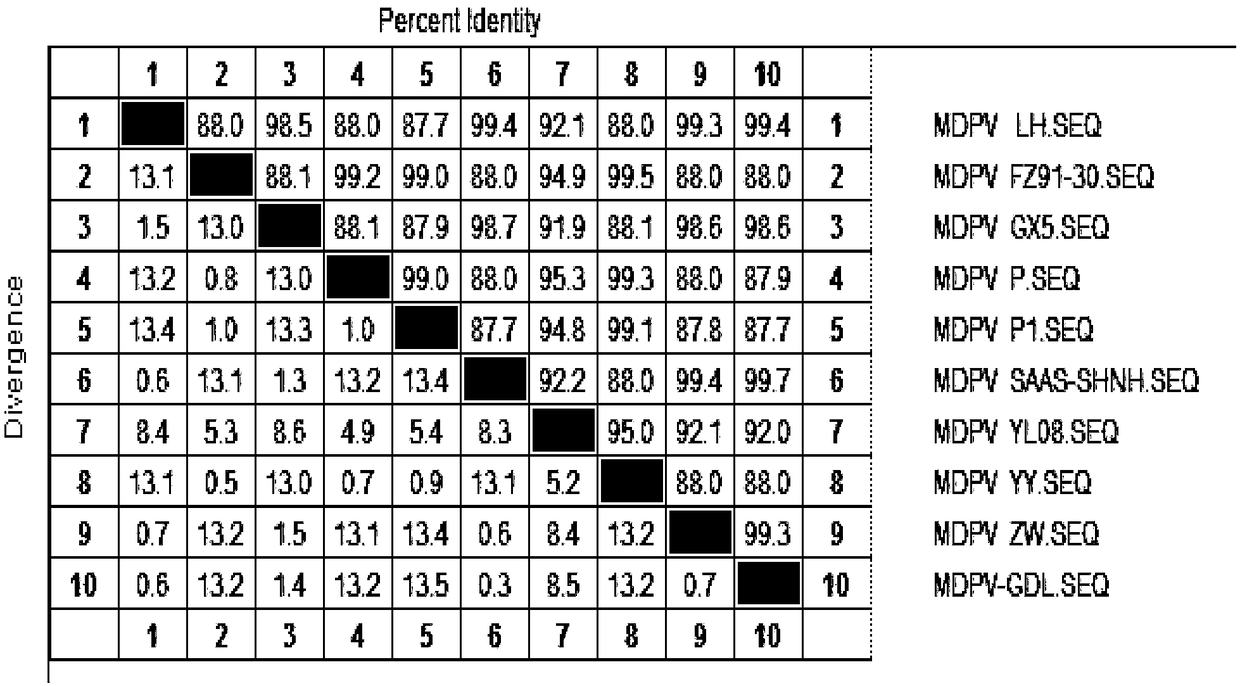
[0019] 1.1.2 Identification of etiology The collected virus liquid was purified and analyzed for virus characteristics in terms ...
Embodiment 2
[0031] Embodiment 2 Vaccine manufacture and semi-finished product inspection
[0032] 2.1 Preparation of virus seeds
[0033] 2.1.1 Preparation of virus seed of GD strain Select muscovy duck embryo fibroblasts that have overgrown a single layer, discard the original culture medium, and add serum-free M199 with a final concentration of 1% virus seed (the third generation of the original virus seed of GD strain) Maintenance solution, static culture at 37°C for 60-78 hours, obvious cytopathy may appear, harvest when the cytopathy reaches more than 80%. According to this method, 10 generations were passed continuously, which were respectively marked as C3-C10 generations. The virus content of each passage was determined. The same generation will be tested for sterility and virus content ≥ 10 6.0 TCID 50 / 0.1ml virus solution, mixed quantitatively, and freeze-dried for storage.
[0034] 2.1.2 Preparation of virus seed of MDPV-GDL strain The allantoic fluid (the third generatio...
Embodiment 3
[0060] Example 3 Vaccine finished product inspection
[0061] 3.1 Traits
[0062] Appearance Milky white emulsion.
[0063] Dosage form water-in-oil type. Take a clean straw, suck a small amount of vaccine and drop it into cold water, except for the first drop, it should not spread.
[0064] Stability Take 10ml of the vaccine and put it into a centrifuge tube, centrifuge at 3000r / min for 15 minutes, the water precipitated at the bottom of the tube should not exceed 0.5ml.
[0065] Viscosity is carried out according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and it should meet the requirements.
[0066] 3.2 Inspection of filling volume Inspection shall be carried out according to the appendix of the current "Chinese Veterinary Pharmacopoeia", and it shall meet the regulations.
[0067] 3.3 Sterility test According to the current "Chinese Veterinary Pharmacopoeia" appendix to test, should be sterile growth.
[0068] 3.4 Safety inspection In order to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com