Immunoregulation DNA vaccine capable of preventing chicken Eimeria maxima
A DNA vaccine, Eimeria technology, applied in medical preparations containing active ingredients, gene therapy, antibody medical ingredients, etc., can solve the problems of pathogenic threat, difficulty in preservation, high cost, etc., and achieve strong immunogenicity , long-lasting, high-safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
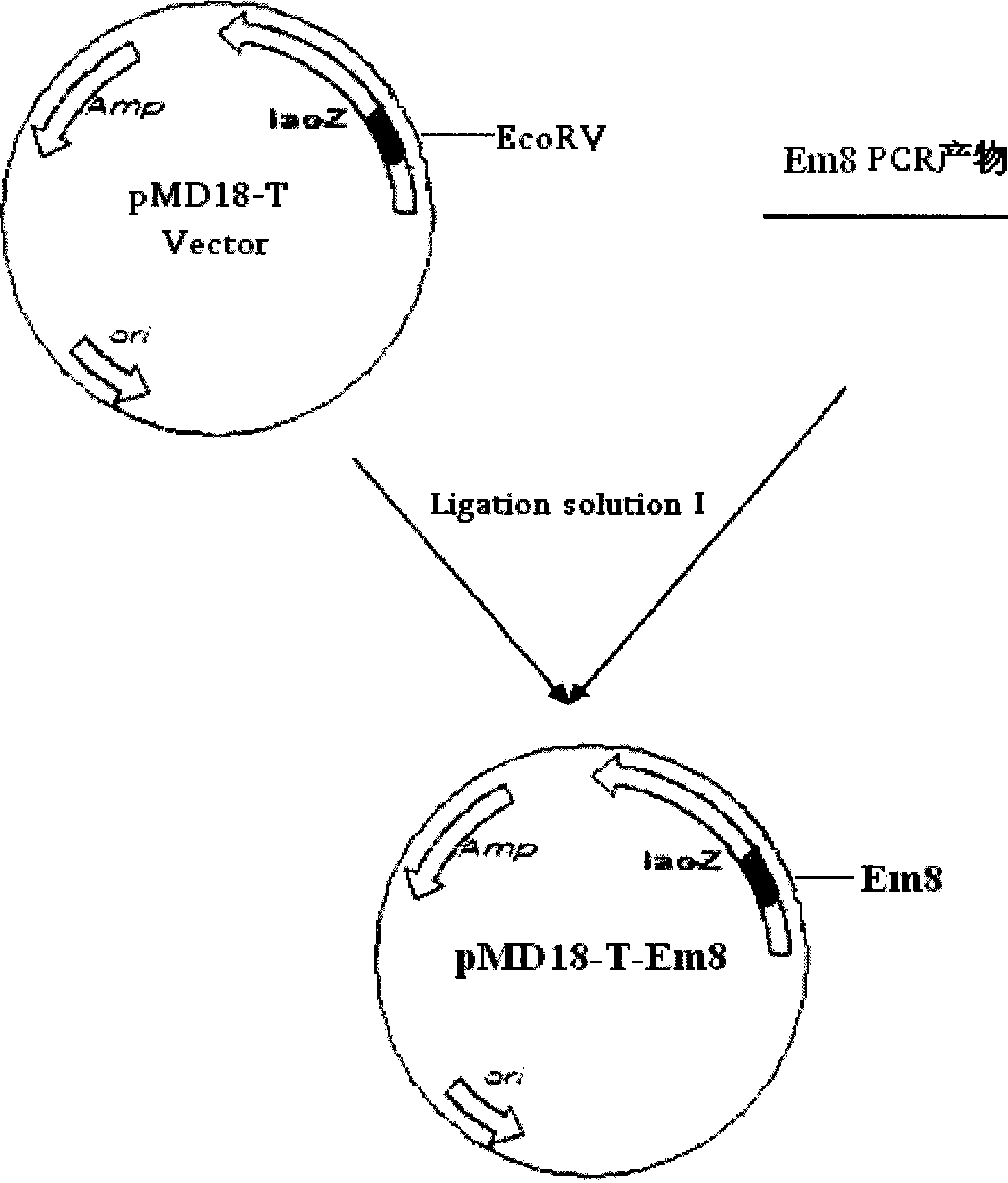
[0036] Preparation of embodiment 1.Em8 gene
[0037] 1.1 Synthetic primers
[0038] DNAStar software was used to analyze the antigenicity and T cell epitope prediction of the giant Emeria antigen gene EmTFP250 (gene accession number AY239227), and select a sequence with a high antigenic index and a relatively concentrated T cell epitope, which was named Em8. The sequence has 1071 nucleotides (bases 1-1071 of SEQ ID NO.1), encodes 357 amino acids, and is located at positions 6240 to 7310 of the EmTFP250 gene. The primers P1 and P2 of the Em8 gene fragment were designed by using the software primerpremier5.0, and sent to TaKaRa to synthesize the primers. The sequence is as follows:
[0039] P1:5′-T AAGCTT ATGCGTGAGGACACCGCT-3';
[0040] P2:5′-A GGATCC CTGAATGTCGCCG-3′
[0041] Among them, the underlined parts are the introduced enzyme cutting sites HindIII and BamHI respectively. To construct recombinant plasmids for tandem cytokines, add coagulation factor X a The s...
Embodiment 2
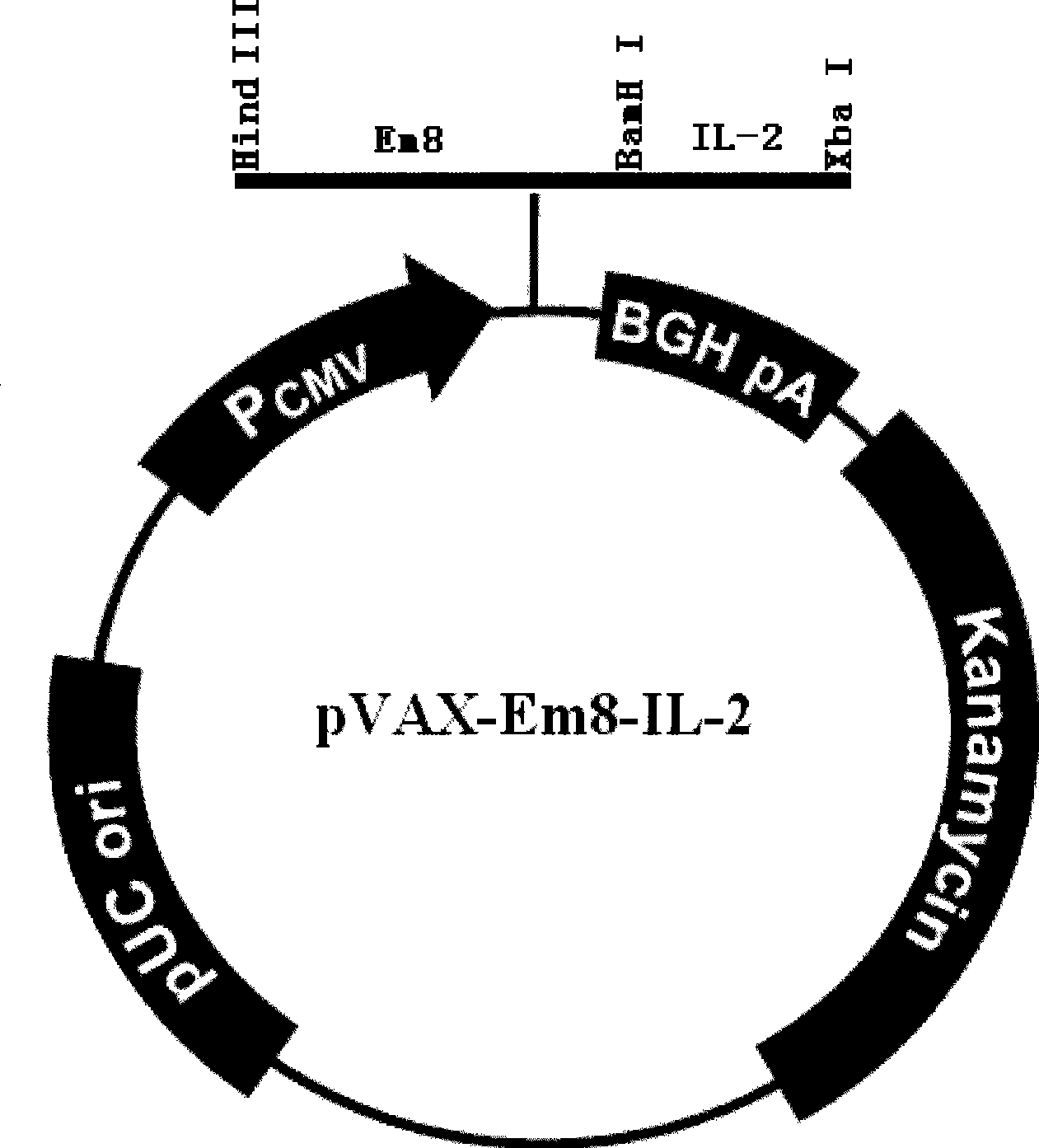
[0052] Example 2. The Construction of Immunomodulatory DNA Vaccine pVAX-Em8-IL-2
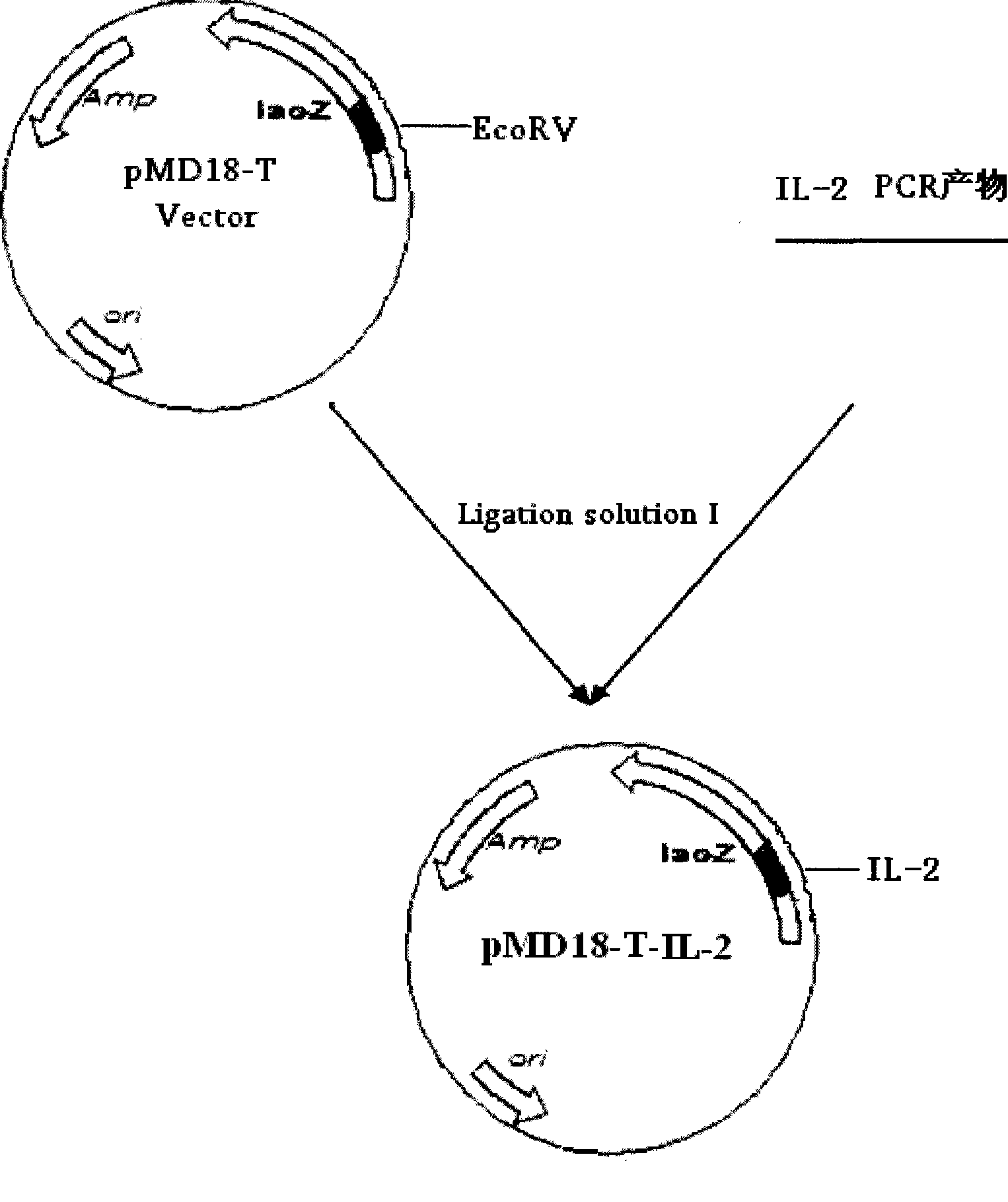
[0053] 2.1 Construction of recombinant plasmid pMD18-T-IL-2
[0054] 2.1.1 Primer synthesis
[0055] According to the published nucleotide sequence of chicken IL-2 (chIL-2) (GenBank accession number AF000631), specific primers P3 and P4 were designed using the software primer premier5.0, and sent to TaKaRa to synthesize primers. The sequence is as follows:
[0056] P3: 5′-CT GGATCC ATGTGCAAAGTACTGAT-3';
[0057] P4: 5′-T TCTAGA TTATTTTTGCAGATATCTCAC-3′
[0058] The underlined parts are the introduced enzyme cutting sites BamHI and XbaI respectively.
[0059] 2.1.2 PCR amplification of IL-2 gene
[0060] Add the following components to a thin-walled PCR tube for PCR amplification:
[0061]
[0062] After centrifuging and mixing the above components, denature on a PCR machine at 94°C for 3 min; 94°C for 45 sec, 60°C for 45 sec, 72°C for 45 sec, a total of 30 cycles; then extend at 72°C ...
Embodiment 3
[0071] Example 3.RT-PCR detection of transcription of vaccine pVAX-Em8-IL-2 in chickens
[0072] A large number of pVAX-Em8-IL-2 recombinant plasmids were extracted, and 14-day-old chicks (100 μg / bird) were injected intramuscularly into the chest, and the injected site (breast muscle) and non-injected muscle (leg) were collected 7 days later. Total muscle RNA was extracted in one step, DNase I was added to remove residual recombinant plasmid and genomic DNA, and the target gene was amplified by RT-PCR. The results showed that the DNA vaccine was transcribed in chicken muscle cells (see Figure 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com