Immunogen and antibody composite vaccine and preparation method thereof
An immunogen and antibody technology, applied in the field of vaccines, can solve the problems of immune efficacy decline and disappearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Preparation of the composite vaccine of Riemeria anatipestifer immunogen and antibody
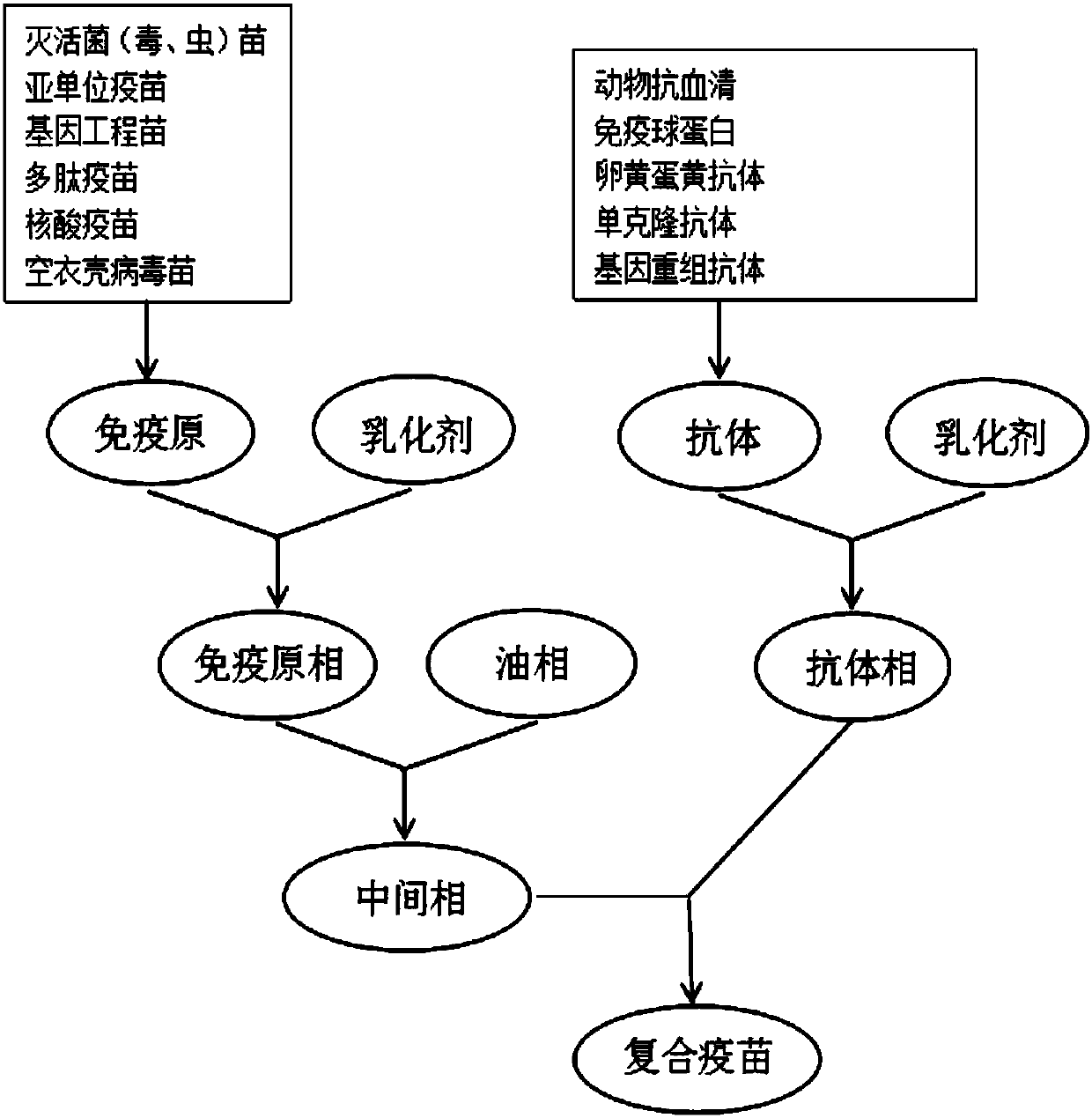
[0080] For the preparation process, see figure 1
[0081] 1) Preparation of the original immunophase: Add sterile Tween 80 at a final concentration of 3.5% (v / v) to the inactivated vaccine solution of Riemeria anatipestifer, and mix well to obtain the original immunology phase.
[0082] 2) Oil phase preparation: add Span 80 at a final concentration of 5% (v / v) to imported white oil, then add aluminum stearate at 3% of the total volume, autoclave for 20 minutes, and mix well That is, the oil phase is obtained.
[0083] 3) Preparation of the antibody phase: Add sterile Tween 80 with a final concentration of 0.9% (v / v) to the refined Riemeria anatipestifer egg yolk antibody solution, and mix well to form the antibody phase.
[0084] 4) Preparation of intermediates: the volume ratio of the immunogen phase to the antibody phase is 1:2. In a sterile environment, start the hom...
Embodiment 2
[0087] Example 2 Preparation of the composite vaccine of Riemeria anatipestifer immunogen and antibody
[0088] 1) Preparation of the original immunophase: Add sterile Tween 80 at a final concentration of 2% (v / v) to the inactivated vaccine solution of Riemeria anatipestifer, mix well to obtain the original immunology phase.
[0089] 2) Oil phase preparation: add Span 80 at a final concentration of 8% (v / v) to imported white oil, then add aluminum stearate at 2.5% w / v of the total volume, and autoclave for 20 minutes. After mixing, the oil phase is obtained.
[0090] 3) Preparation of antibody phase. Add sterile Tween 80 with a final concentration of 0.6% (v / v) to the refined Riemeria anatipestifer egg yolk antibody solution, and mix well to form the antibody phase.
[0091] 4) Preparation of intermediates: the volume ratio of the immunogen phase to the antibody phase is 1:2.3. Under a sterile environment, start the homogenizer, add the oil phase into the emulsification tan...
Embodiment 3
[0094] Example 3 Preparation of the composite vaccine of Riemeria anatipestifer immunogen and antibody
[0095] 1) Preparation of the original immunological phase: Add sterile Tween 80 at a final concentration of 4% (v / v) to the inactivated vaccine solution of Riemeria anatipestifer, and mix well to obtain the original immunological phase.
[0096] 2) Preparation of oil-free phase: use imported oil adjuvant and directly use it as oil phase.
[0097] 3) Preparation of antibody phase. Add sterile Tween 80 with a final concentration of 1.8% (v / v) to the refined Riemeria anatipestifer egg yolk antibody solution, and mix well to form the antibody phase.
[0098] 4) Preparation of intermediates: the volume ratio of the immunogen phase to the antibody phase is 1:3. In a sterile environment, start the homogenizer, add the oil phase into the emulsification tank, adjust the speed to 6500 rpm, and slowly add the original immune phase while stirring. After the addition of the immunogen p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com