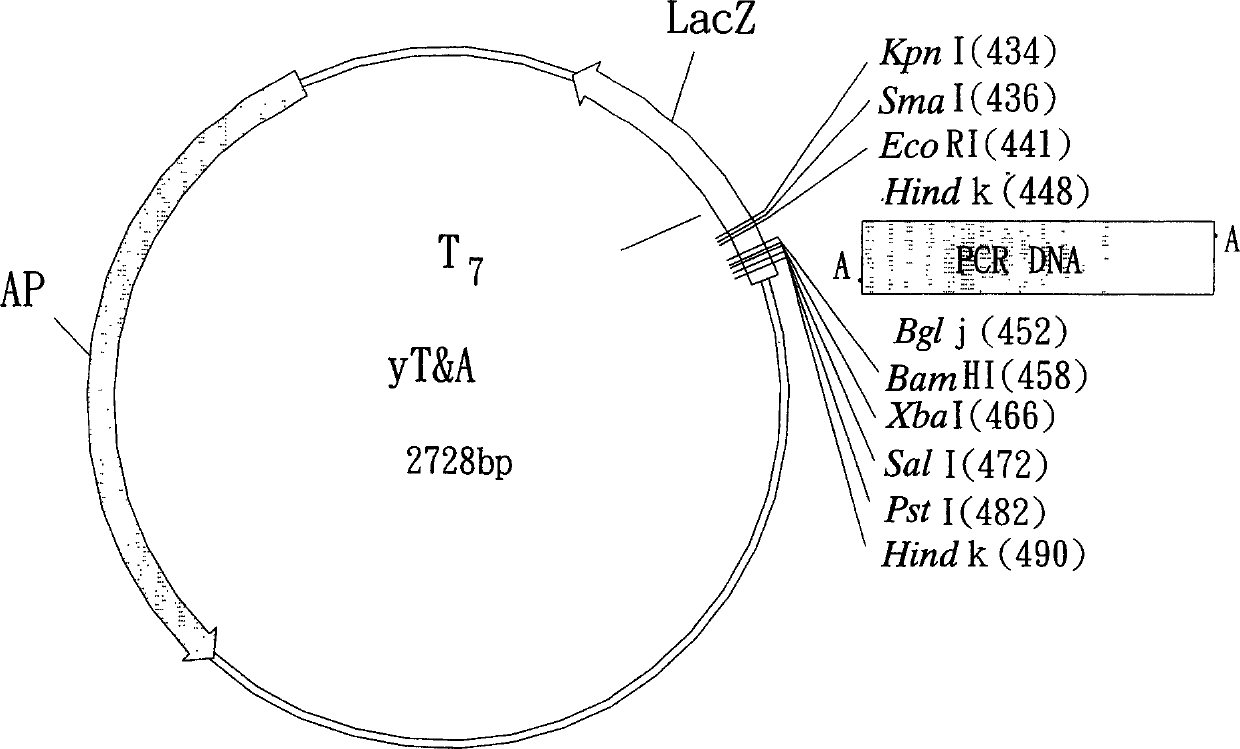
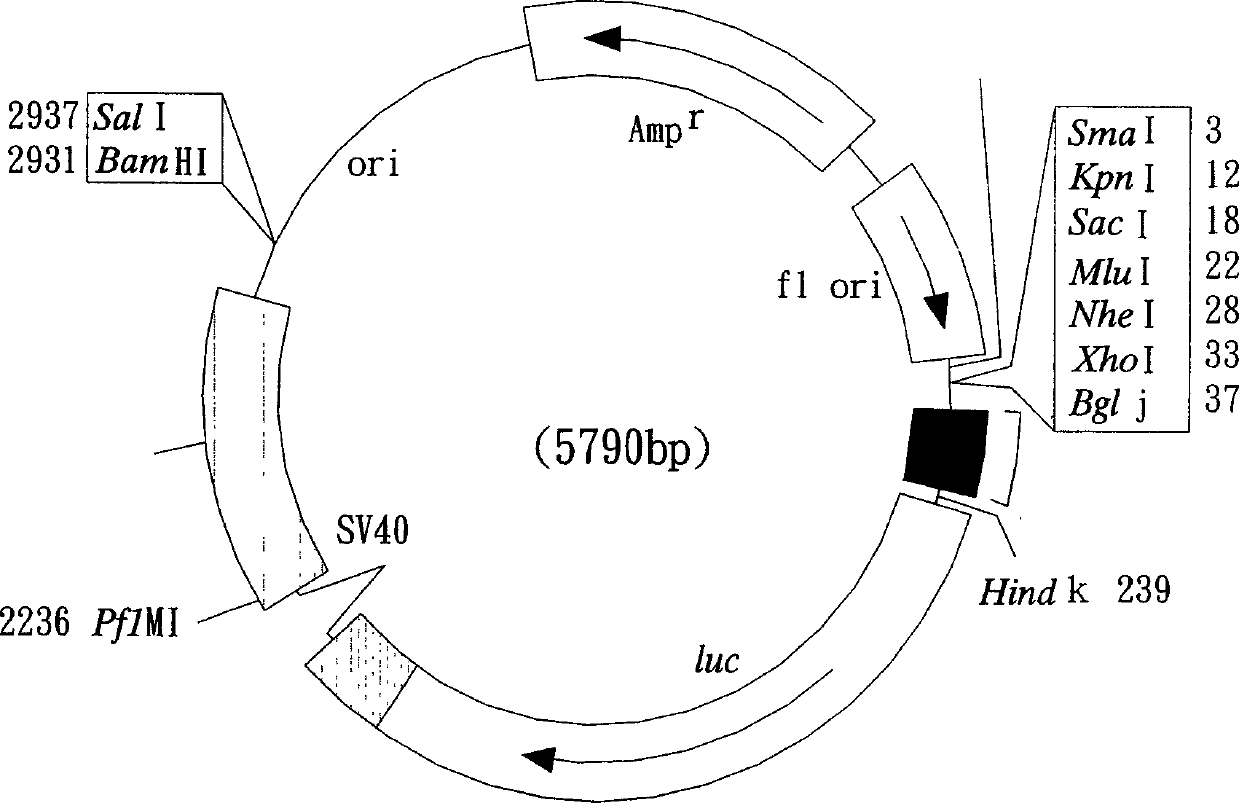
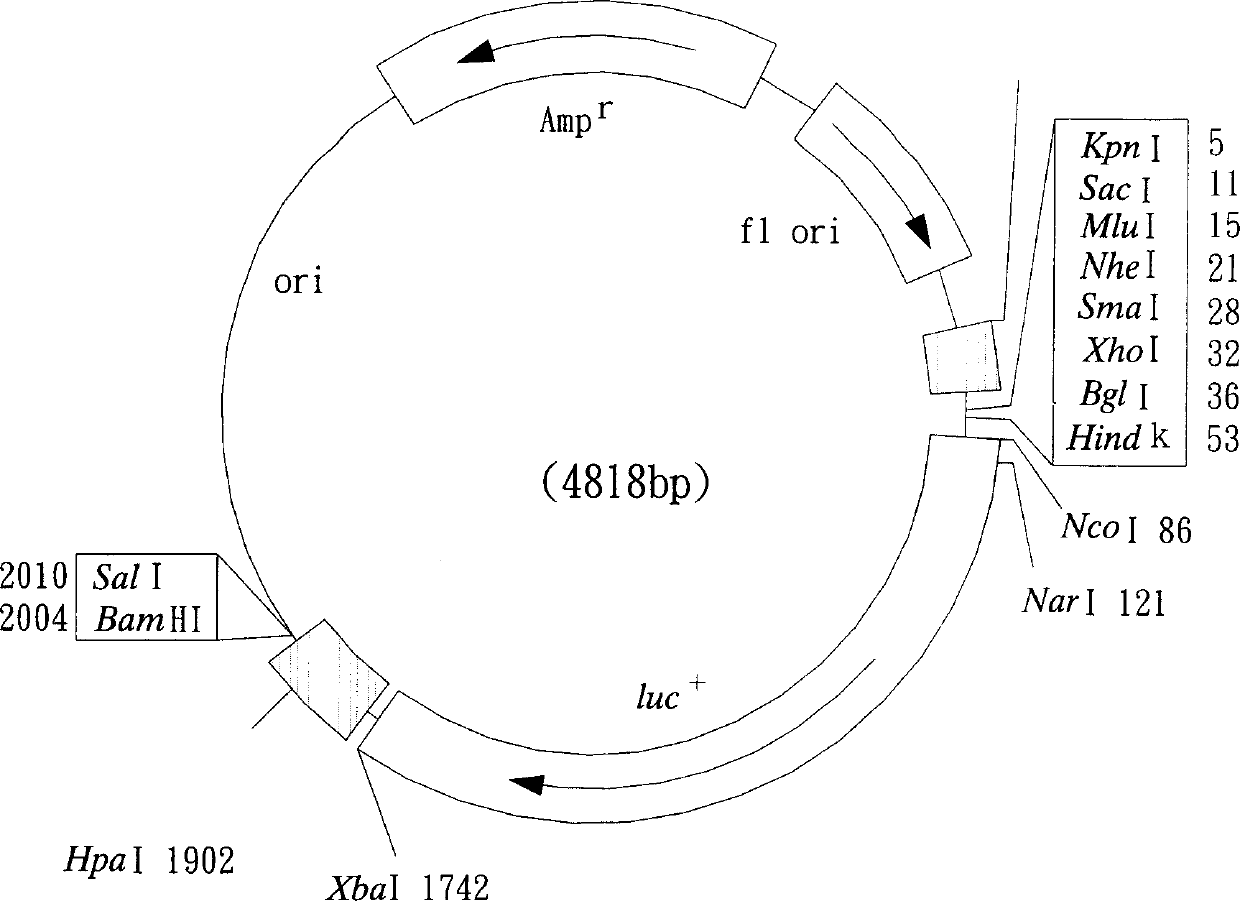
Recombinant cell strain for detecting dioxin compounds according to behaviour of leuciferinase gene
A technology of recombinant cells and recombinant vectors, which can be applied to cells modified by introducing foreign genetic material, recombinant DNA technology, and the determination/inspection of microorganisms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1. Construction of recombinant plasmids M4P2 and MP containing dioxin-response elements containing dioxin-response elements
[0146] According to the nucleotide sequence shown in the accession number (accession number) AF210905 [Mus musculus (Mus musculus) CYP1A1 gene, promoter region and partial sequence] registered in the NCBI website and the accession number X01681 [Mus musculus (Mus musculus) musculus) CYP1A1 gene], and use the software Primer3 Version to design the following two sets of primer pairs for the dioxin-reactive fragments (DREs) in the Mus musculus (Mus musculus) CYP1A1 gene sequence (Mus musculus) CYP1A1 gene sequence ( primer pairs), wherein the nucleotide sequences of these primers were synthesized by Bost Biotechnology Co., Ltd.:
[0147] Forward primer 1
[0148] 5'-cagagagcacctgcaaaaca-3' (SEQ ID NO: 1)
[0149] Reverse primer 1
[0150] 5'-ggctacaaagggtgatgctt-3' (SEQ ID NO: 2)
[0151] forward primer 2
[0152] 5'- ctcgag gagggcaggt...
Embodiment 2
[0172] Example 2. Transfection of mouse hepatoma cells Hepa-1C1C7 with recombinant vectors M4P2 and MP using recombinant vectors M4P2 and MP
[0173] The Hepa-1C1C7 cell line can be transfected with the recombinant vector MP or M4P2 according to the following preparation procedure A or preparation procedure B, wherein the culture method of the Hepa-1C1C7 cell line is as described in the section "Cell Lines and Culture Conditions" above operating procedures.
[0174] Preparation Procedure A:
[0175] 1. Mix 172 μl of basic DMEM with 28 μl of GenePORTER TM 2. Mix Transfection Reagent (GST Inc.) evenly to form a first solution, and add 200 μl of New DNA diluent B (GST Inc.) and 8 μg of DNA [wherein the selected recombinant vector DNA and pcDNA (InvitrogenCooperation) are each 4 μg ] mixing uniformly to form a second solution, and allowing the first and second solutions to stand separately for 5 minutes; then, mixing the two solutions uniformly, and then allowing the resulting ...
Embodiment 3
[0190]Example 3. Establishment of the correlation of the correlation of dioxin concentration vs. the light intensity caused by luciferase with respect to the light intensity caused by luciferase
[0191] A. Expression of luciferase gene in transfected cells after the stimulation of dioxin:
[0192] The transfected cells (4×10 5 cells / well) were placed in a 24-well culture plate, and 1ml of medium (DMEM+10%FCS) was added to each well, and then the culture plate was placed at 37°C, 5% CO 2 placed in a constant temperature incubator for 2 hours to allow the cells to attach.
[0193] Dioxin (2,3,7,8-TCDD) stock solution (stock solution) with a concentration of 10 μg / ml was made 10-fold serial dilution (10-fold serial dilution) with DMSO, and 3.22 μl was taken for each dilution And added to each well of the culture plate, so that the final concentration of dioxin in each experimental group was 10nM, 1nM, 100pM, 50pM, 10pM, 1pM and 0.1pM, and each experimental group was repeated 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com