CA16 infectious clone with green fluorescent protein gene as well as construction method and application of CA16 infectious clone
A green fluorescent protein, infectious cloning technology, which is applied in the application field of eGFP-CA16 reporter virus, can solve the problems of lag in vaccine and therapeutic drug research, lack of epidemiological research data, etc., to avoid operational difficulties and ensure fidelity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A kind of preparation that has the CA16 infectious clone of green fluorescent protein gene, its steps are:
[0056] 1. Extraction of CA16 / GD09 / 24 viral RNA:
[0057] Take 140ul parental CA16: CA16 / GD09 / 24 virus solution (from Jian-Feng Han, Nan Yu, Yu-Xian Pan, Si-Jie He, Li-Juan Xu, Rui-Yuan Cao, Yue-Xiang Li, Shun- Ya Zhu, Yu Zhang, E-De Qin, Xiao-Yan Che, Cheng-Feng Qin. Phenotypic and genomic characterization of humancoxsackievirus A16 strains with distinct virulence in mice. Virus Re s .2014Jan22;179:212-9. According to the requirements of the QIAamp Viral RNA Mini Kit (purchased from Qiagen) kit instructions, CA16 RNA was extracted and stored at -80°C for future use;
[0058] 2. Segmented amplification of the genome sequence of the CA16 / GD09 / 24 virus strain:
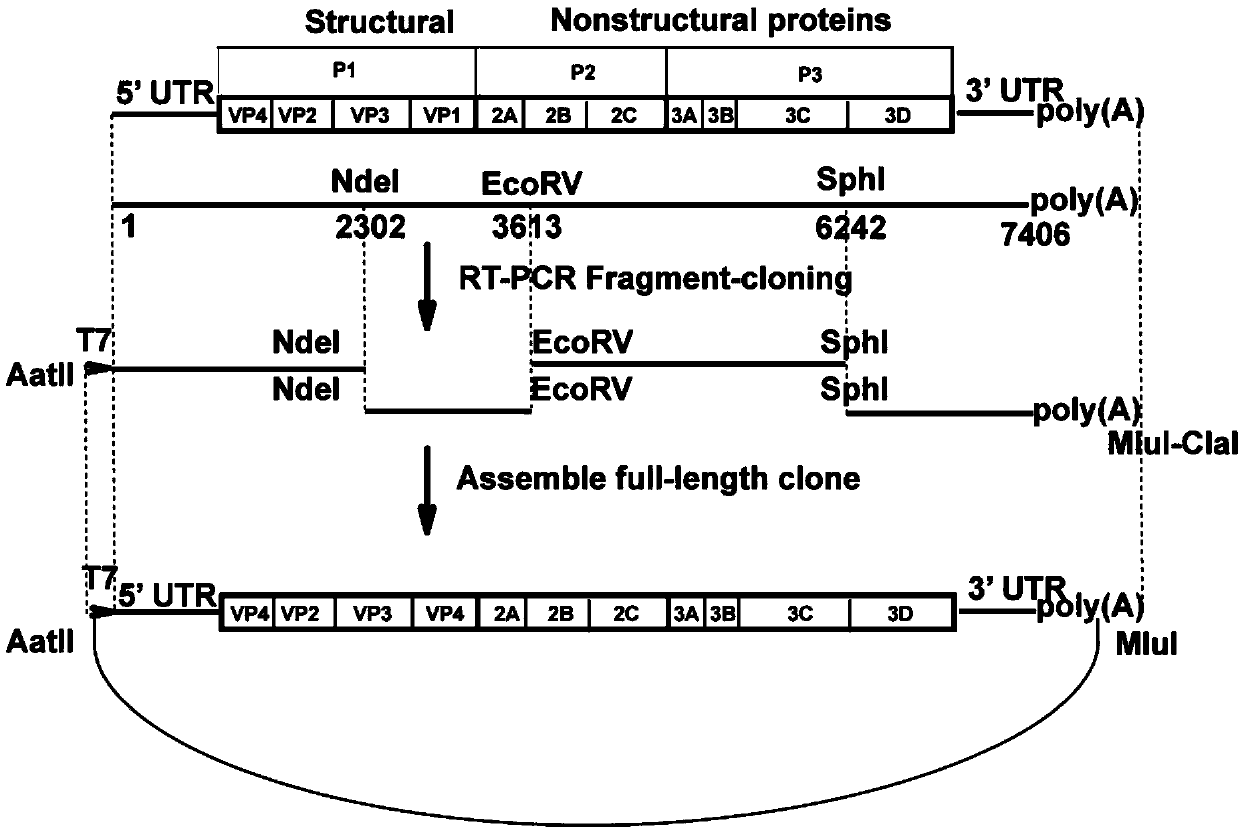
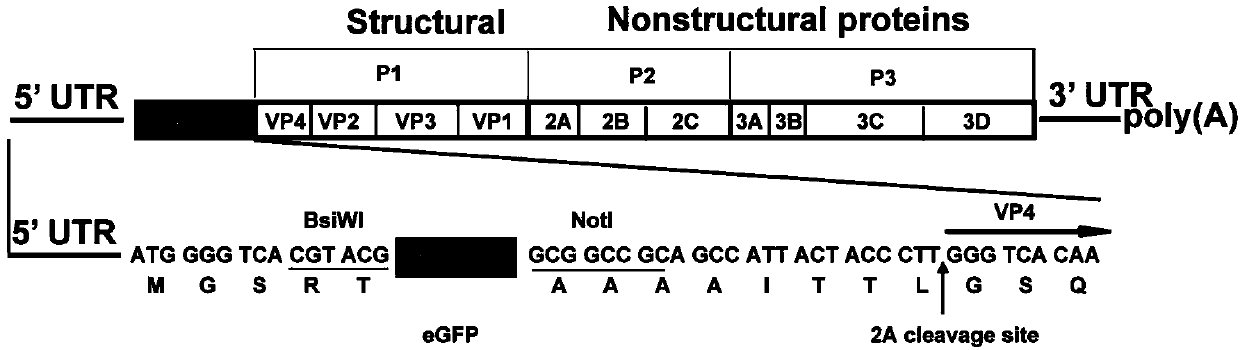
[0059] Construct a cDNA infectious clone with the full-length genome of CA16: According to the sequence of the CA16 / GD09 / 24 virus strain (GenBank accession no.KC117317), use the viral RNA in step 1 as a ...
Embodiment 2
[0072] The CA16 infectious clone with the green fluorescent protein gene successfully rescued the virus with the same growth tendency as the recombinant CA16 virus:
[0073] 1. Linearization and phenol-chloroform extraction of plasmids: 10 μg of plasmid pACYC177-CA16-FL and plasmid pACYC177-eGFP-CA16-FL were digested with MluI (described above), digested at 37°C for two hours, and washed with 0.8% agar After sugar gel electrophoresis to identify the complete digestion, add 100 μl saturated phenol (purchased from Sinopharm Chemical Reagent Company) to the digested product, shake and mix, centrifuge at 17000g for 5min, draw the supernatant into a new centrifuge tube, and centrifuge to the original Add 100 μl sterile water to the tube, shake and mix well, and centrifuge at 17,000 g for 5 minutes; absorb the supernatant and combine it with the supernatant obtained in the previous step (total volume is about 200 μl), add 200 μl chloroform (purchased from Sinopharm Chemical Reagent C...
Embodiment 3
[0083] A kind of application of CA16 infectious clone with green fluorescent protein gene in antiviral drug screening, its steps are:
[0084] 1. Inoculate 1×10 cells in each well of a 12-well cell culture plate 5 vero cells at 37°C, 5% CO 2 Under culture conditions, when the confluence reaches 60%, according to the multiplicity of infection (MOI) of 0.1, add 200 μl of diluted parental CA16, recombinant CA16 and eGFP-CA16 virus solution to each well, 37 ° C, 5% CO 2 After adsorption in the incubator for 1 hour, the virus solution was discarded, and 1ml of DMEM medium containing 2% fetal bovine serum was added to each well;
[0085] 2. Dilute the stored NITD008 at a concentration of 20mM to 0, 1, 2, 3, 4mM with DMSO, and add 1μl of diluted NITD008 to the wells respectively, that is, the final concentrations are 0, 1, 2, 3, 4μM respectively . 37°C, 5% CO 2 cultured in an incubator for 48 hours, and observed the expression of eGFP under the action of different concentrations ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com