Broad-spectrum anti-enterovirus protein drug and application thereof
An enterovirus and protein technology, applied in the field of biomedicine, can solve the problem of inability to cross-protect infection, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] Methods for preparing formulations known in the art can be found, for example, in "Remington's Pharmaceutical Sciences" (19th Ed.), Ed. A. Gennaro, 1995, Mack Publishing Company, Easton, Pa. Formulations for parenteral administration may, for example, contain excipients, sterile water or saline, poly(alkylene) glycols such as polyethylene glycol, vegetable oils or hydrogenated naphthalenes. Biocompatible, biodegradable lactide polymers, lactide / glycolide copolymers, or polyoxyethylene-polyoxypropylene copolymers can be used to control the release of the compounds. Other potentially useful parenteral delivery systems include ethylene-vinyl acetate copolymer particles, osmotic pumps, implantable infusion systems, and liposomes. Formulations for inhalation may contain excipients such as lactose, or may be aqueous solutions containing, for example, polyoxyethylene-9-lauryl ether, glycocholate and deoxycholate, or may be in the form of nasal drops or Oily solution applied a...
Embodiment 1
[0068] Example 1: Preparation of protein modified by acid anhydride
[0069] 3-hydroxy-phthalic anhydride-modified human serum albumin (3-hydroxyphthalic anhydride-modified human serum albumin, 3HP-HSA) and 3-hydroxy-phthalic anhydride-modified bovine β-lactoglobulin (3- Preparation of hydroxyphthalic anhydride-modified bovine beta-lactoglobulin, 3HP-β-LG):
[0070] (1) Prepare 0.1M disodium hydrogen phosphate, adjust the pH to 8.5, and filter through a 0.2 μm filter membrane to sterilize.
[0071] (2) Dissolve human serum albumin (HSA) and bovine β-lactoglobulin (β-LG) powders in the prepared disodium hydrogen phosphate solution to make the concentration 20 mg / ml.
[0072] (3) Acid anhydrides (3-hydroxy-phthalic anhydride (3HP), maleic anhydride (ML), succinic anhydride (SU)) were dissolved in DMSO to a concentration of 1M.
[0073] (4) An acid anhydride with a final concentration of 12 mM was added to the human serum albumin and bovine β-lactoglobulin solution. Mix immedi...
Embodiment 2
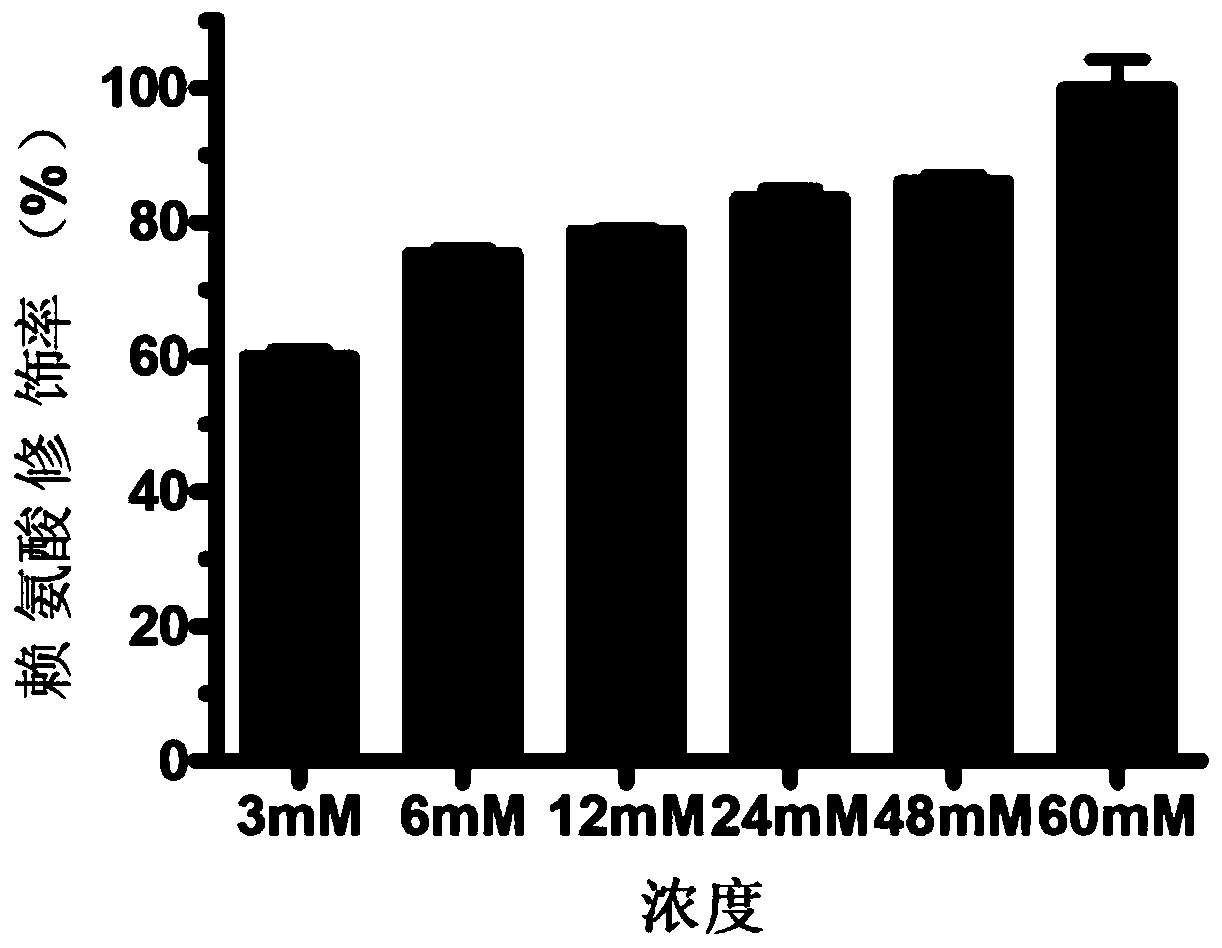
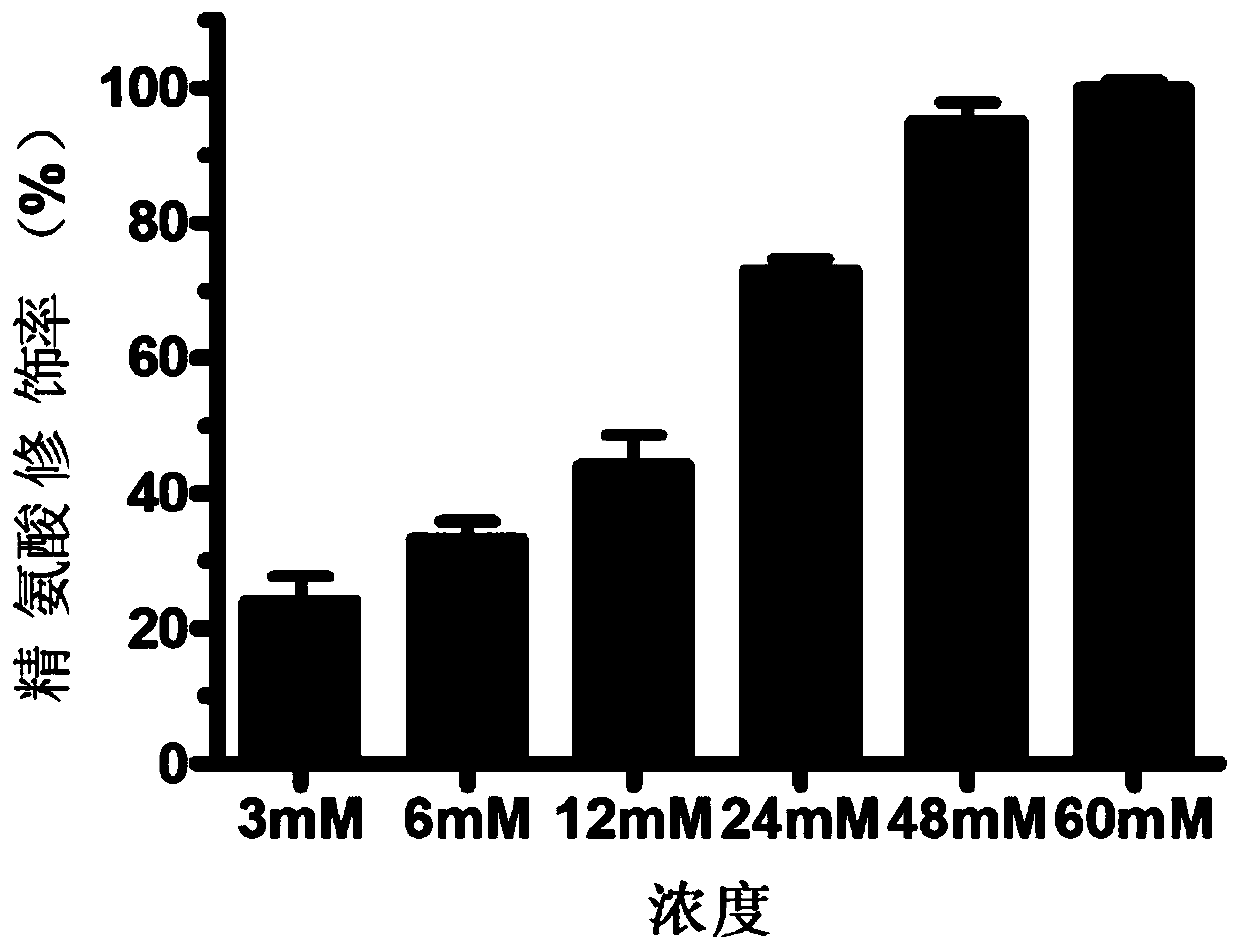
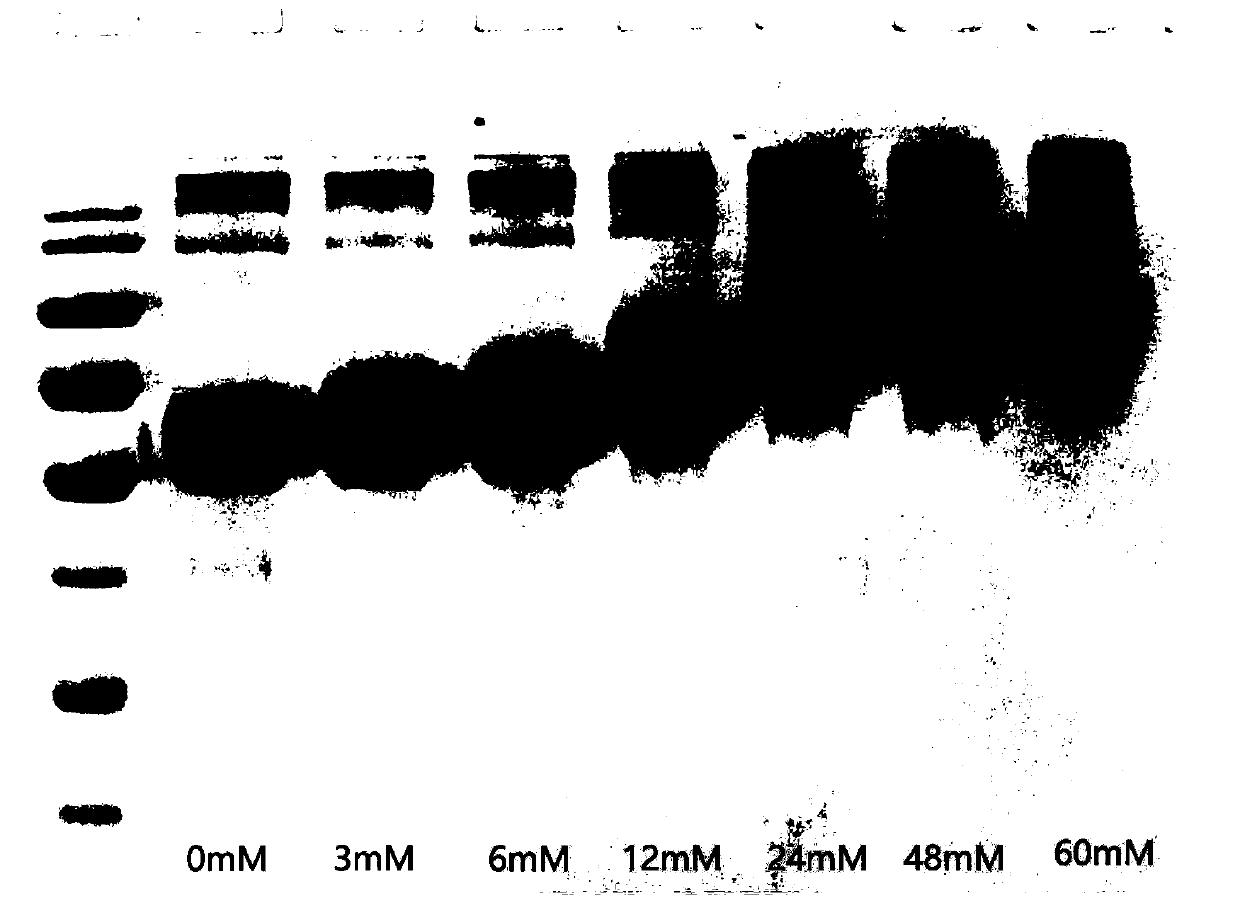
[0081] Example 2: Preparation of proteins with different anhydride modification rates
[0082] (1) Dissolve 20 mg of human serum albumin in 1 ml of 0.1 M disodium hydrogen phosphate solution.
[0083] (2) 3-Hydroxy-phthalic anhydride was dissolved in DMSO to form a 1M mother liquor.
[0084] (3) Add 0mM, 3mM, 6mM, 12mM, 24mM, 48mM and 60mM 3-hydroxy-phthalic anhydride to the human serum albumin solution, respectively.
[0085] (4) Adjust the pH to 8.5-9.0 with 5M sodium hydroxide.
[0086] (5) React at room temperature for 2 hours.
[0087] (6) The modified protein was quantified using the BCA kit of Takara Company. Refer to the manual for specific operations.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com