Prodrugs of substituted polycyclic carbamoyl pyridone derivatives
A technology of substituents and alkyl groups, applied in the fields of polycyclic carbamoyl pyridone derivatives, prodrugs thereof and pharmaceutical compositions containing them, can solve the problem that there is no record of cap-dependent endonuclease, no record of previous medicine and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
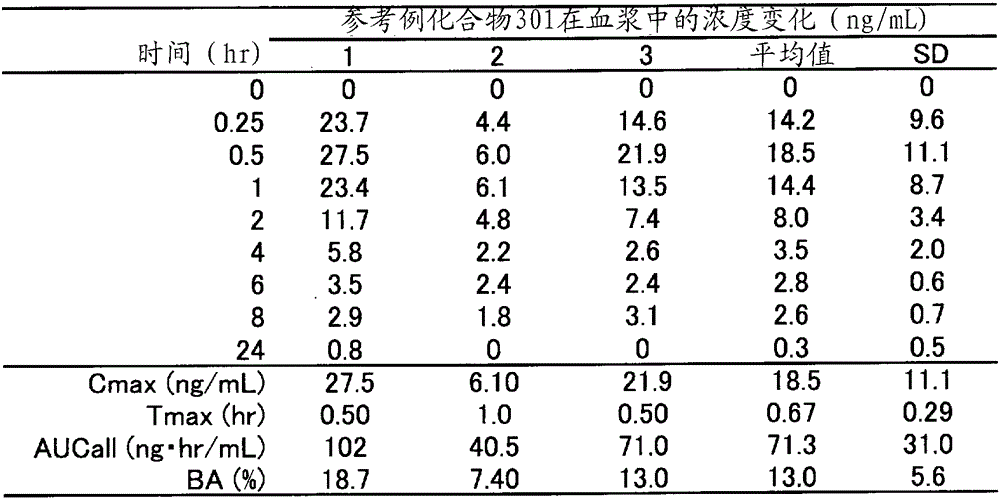
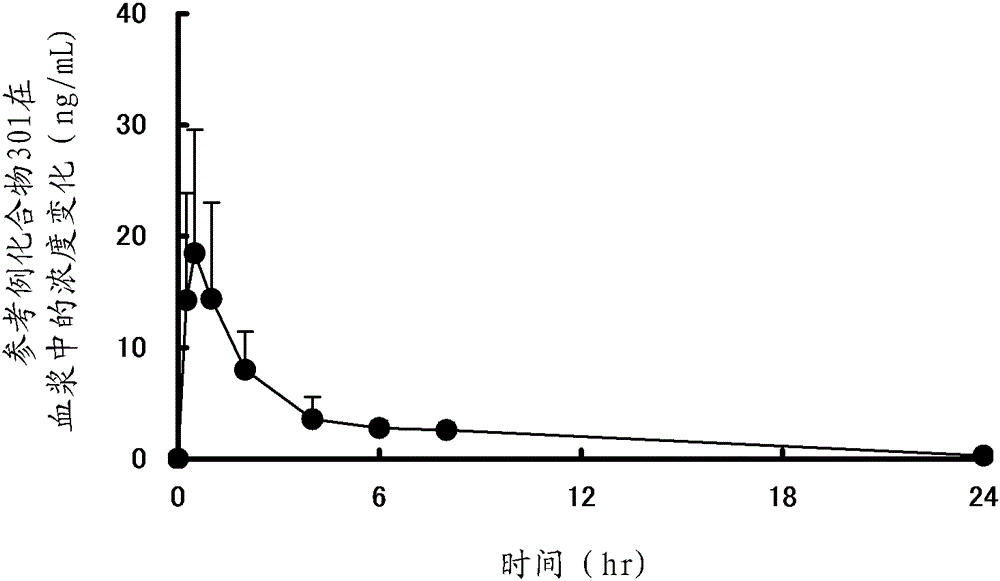
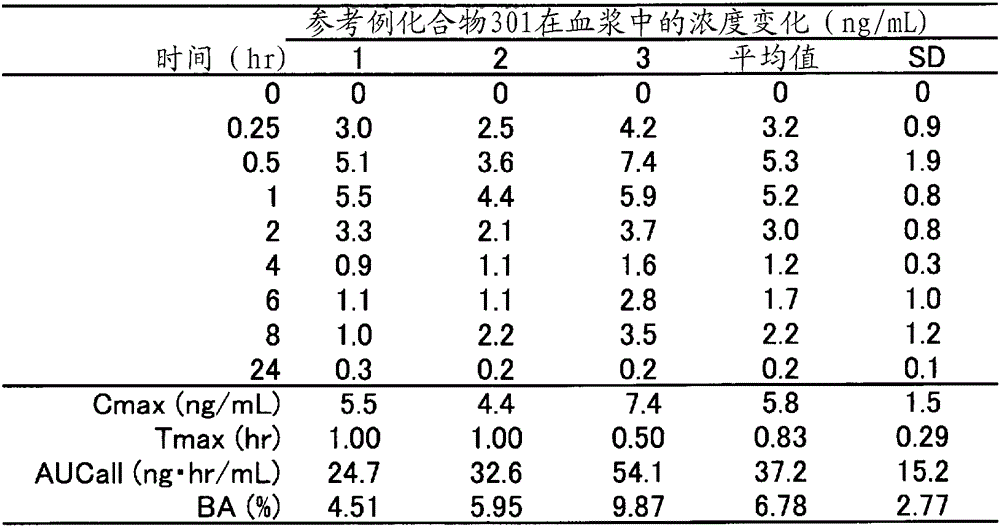
reference example 1
[1309] 【Chemical 79】
[1310]
[1311] first step
[1312] A solution of compound 1A (12.8 g, 89.4 mmol) and pyridine (8.50 g, 107 mmol) in dichloromethane (90 mL) was cooled to 1-3° C., and benzyloxyacetyl chloride was added dropwise over 50 minutes while maintaining the same temperature ( 19.8 g, 107 mmol) in dichloromethane (90 mL). After stirring the reaction solution at the same temperature for 30 minutes, the temperature was gradually raised to 15° C. over 60 minutes, and ice water was added. The dichloromethane layer was separated, and the aqueous layer was extracted once with dichloromethane. The combined extracts were washed three times with water, washed with saturated brine, and then dried. The solvent was distilled off, and the obtained oil was purified by silica gel column chromatography. It was first eluted with n-hexane, followed by n-hexane-ethyl acetate (1:1, v / v). When the target fraction was concentrated, 22.2 g of Compound 1B was obtained as an oil....
reference example 2
[1339] 【Chemical 80】
[1340]
[1341] first step
[1342] Trifluoroacetic acid (40 ml) was added to (S)-tert-butyl 3-hydroxy-1,1-diphenylpropan-2-ylcarbamate (5.00 g, 15.3 mmol), followed by stirring under ice-cooling for 1 hour. After trifluoroacetic acid was distilled off, toluene was added and the mixture was again distilled off under reduced pressure to obtain crude (S)-2-amino-3,3-diphenylpropan-1-ol. To the obtained (S)-2-amino-3,3-diphenylpropan-1-ol, compound 1F (5.73 g, 15.3 mmol), toluene (50 ml), triethylamine (6.4 ml, 45.8 mmol) were added , stirred at 90° C. for 1 hour, and cooled to room temperature, and then the solvent was distilled off. Dichloromethane was added to the obtained residue, followed by washing with 2N aqueous hydrochloric acid solution, saturated aqueous sodium bicarbonate solution, and saturated brine. After separating the organic layer, magnesium sulfate was added, Celite filtration was performed, and the filtrate was distilled off to obt...
reference example 3
[1357] 【Chemical 81】
[1358]
[1359] According to Reference Example 2, compound 3 was synthesized by the same method.
[1360] 1 H-NMR (DMSO-d 6 )δ: 3.15(1H, m), 3.26(3H, s), 3.52-3.70(4H, m), 3.70-3.80(2H, m), 4.10(1H, d, J=12.9Hz), 4.92(1H , brs), 6.98(1H, t, J=7.4Hz), 7.03(1H, brs), 7.08(1H, t, 7.6Hz), 7.34(1H, d, J=7.8Hz), 7.47(1H, d , J=7.3Hz), 7.80 (1H, s), 10.94 (1H, brs), 15.38 (1H, brs).
[1361] MS: m / z = 412.4 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com