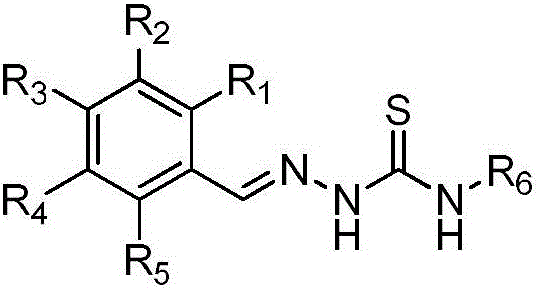
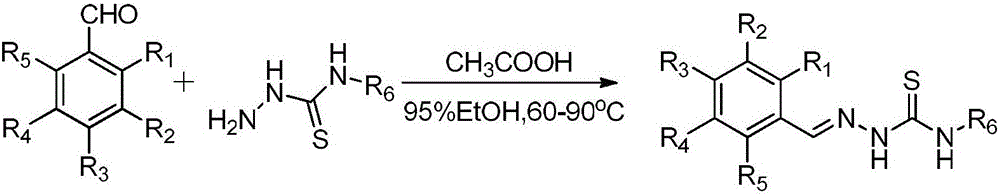
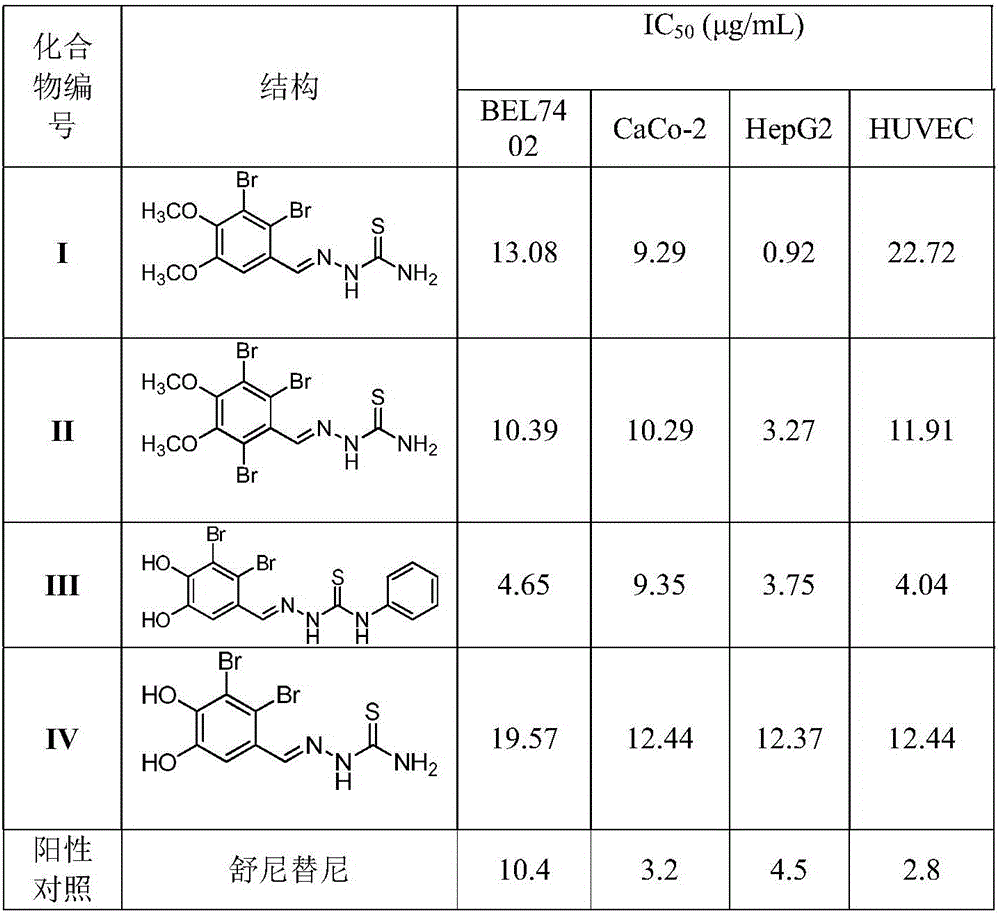
Novel bromophenol amino thiourea compounds and preparation, drugs, and applications thereof
A technology of bromophenol thiosemicarbazide and bromophenol amino, which is applied in the fields of bromophenol thiosemicarbazide compounds I-IV and their pharmacological activities and pharmaceutical applications, can solve the problems such as no reports of bromophenol thiosemicarbazide compounds, and achieve normal cell Low toxicity, good antitumor activity and selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: 2-(2,3-dibromo-4,5-dimethoxybenzylidene)thiosemicarbazide (2-(2,3-dibromo-4,5-dimethoxybenzylidene)hydrazinecarbothioamide, compound I ) preparation
[0026] Weigh 10mmol 2,3-dibromo-4,5-dimethoxybenzaldehyde (3.22g) and 11mmol thiosemicarbazide (1g) into a 500mL reaction flask, add 30mL ethanol (95%) and stir evenly, Add 5mL of glacial acetic acid dropwise, then reflux and stir at 65-70°C for about 10 hours, evaporate most of the ethanol under reduced pressure, add 20ml of ice water, filter to obtain a precipitate, wash the precipitate with ice water (30mL divided into three times), and recrystallize from ethanol to obtain 2-( 2,3-Dibromo-4,5-dimethoxybenzylidene)thiosemicarbazide, white solid, yield 96%; 1 H-NMR (500MHz, DMSO- d6 )δ: 11.62(1H,s), 8.44(1H,s), 8.34(1H,s), 8.20(1H,s), 7.80(1H,s), 3.91(3H,s), 3.76(3H,s ); 13 C-NMR (125MHz, DMSO- d6 )δ: 178.5, 153.0, 148.9, 142.1, 131.2, 121.6, 117.5, 110.6, 60.7, 57.1; ESIMS: m / z 393 [M-H] — HRESIMS: calc...
Embodiment 2
[0027]Example 2: 2-(2,3,6-tribromo-4,5-dimethoxybenzylidene)thiosemicarbazide (2-(2,3,6-tribromo-4,5-dimethoxybenzylidene) hydrazinecarbothioamide, compound II)
[0028] The preparation method of compound II is similar to the preparation method of compound I, and its difference from Example 1 is that the raw material 2,3-dibromo-4,5-dimethoxybenzaldehyde is replaced by 2,3,6-tri Bromo-4,5-dimethoxybenzaldehyde, compound II was prepared as a white solid with a yield of 92%, 1 H-NMR (600MHz, DMSO- d6 )δ: 11.72(1H,s), 8.32(1H,s), 8.07(1H,s), 7.56(1H,s), 3.86(3H,s), 3.83(3H,s); 13 C-NMR (150MHz, DMSO- d6 )δ: 179.0, 152.5, 151.1, 141.8, 132.5, 122.0, 121.5, 119.5, 61.6, 61.5; ESIMS: m / z 471 [M-H] — HRESIMS: calcfor C10H10N3O2S Br3[M-H] — 471.7971, found 471.7960.
Embodiment 3
[0029] Example 3: 2-(2,3-dibromo-4,5-dihydroxybenzylidene)-N-phenylthiosemicarbazide (2-(2,3-dibromo-4,5-dihydroxybenzylidene)- N-phenylhydrazinecarbothi oamide, compound III)
[0030] The preparation method of compound III is similar to the preparation method of compound I, and its difference from Example 1 is that the raw material 2,3-dibromo-4,5-dimethoxybenzaldehyde is replaced by 2,3-dibromo- 4,5-dihydroxybenzaldehyde, compound III was prepared as a white solid with a yield of 78%, 1 H-NMR (600MHz, DMSO- d6 )δ: 11.94(1H,s), 10.16(2H,s), 10.03(1H,s), 8.50(1H,s), 7.73(1H,s), 7.60(2H,d,J=7.8Hz), 7.37(2H,dd,J=7.8,6.0Hz),7.19(1H,dd,J=7.2,7.2Hz); 13 C-NMR (150MHz, DMSO- d6 )δ: 176.4, 147.7, 145.9, 143.6, 128.7 (2C), 126.0, 125.8 (3C), 117.0, 113.8, 113.4; ESIMS: m / z 441 [M-H] — HRESIMS: calcfor C14H11N3O2SBr2[M-H] — 441.8866, found 441.8848.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com