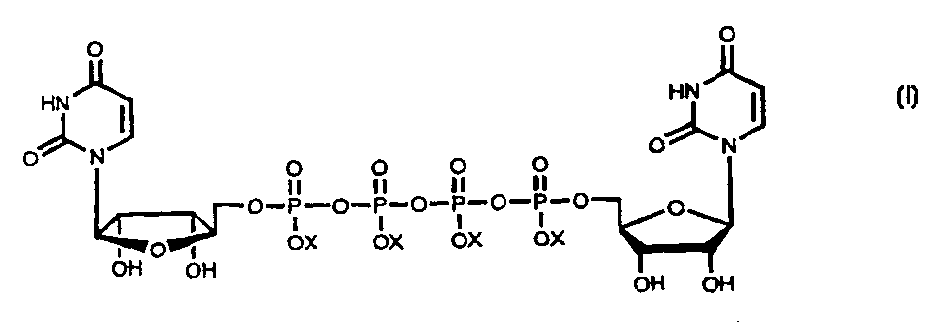
Method for large-scale production of Di (uridine 5'-tetraphosphate) and salts thereof
A technology of tetraphosphate and uridine, which is applied in the field of production of therapeutic dinucleotides, can solve problems such as time-consuming, and achieve the effect of reducing time and being easy to handle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Method for producing diuridine tetraphosphate tetrasodium salt with uridine 5'-diphosphate
[0047] Uridine 5'-diphosphate disodium salt (Yamasa, Choshi, Japan; 600 g) was dissolved in 5.4 liters of deionized water. Pass the solution through Dowex 50Wx4 H + (Dow Chemical) column. Fractions containing uridine 5'diphosphate were pooled and neutralized with tributylamine (Aldrich, St. Louis; 300 mL). The neutralized fractions were concentrated to an oil using a rotary evaporator at a bath temperature of 55-60°C. The oil was dissolved in dry dimethylformamide (Aldrich, 3 L) and concentrated to an oily dryness using a rotary evaporator (55-60°C bath temperature). Repeat this step twice. The oil was redissolved in 3 liters of dimethylformamide and 1,1-carbonyldiimidazole (Aldrich; 100 g) was added. The solution was heated at 50°C for 2.5 hours. An additional amount of activator (33 g) was added and heating was continued for an additional 2.5 hours. The solution was con...
Embodiment 2
[0054] Method for producing diuridine tetraphosphate tetrasodium salt with uridine 5'-monophosphate
[0055] Uridine 5'-monophosphate (Sigma, Milwaukee, 3.0 g, 9.26 mmol) was dissolved in dry DMF (10 mL) and tributylamine (Aldrich, 2 mL). The solution was evaporated in vacuo at 40°C to an oil. The residue was dissolved in dry DMF (Aldrich, 8 mL) to form a solution. To this solution was added carbonyldiimidazole (Aldrich, 1.65 g, 10.18 mmol). The reaction solution was heated at 50°C for 1 hour. Uridine 5'-triphosphate (Yamasa, 5.60 g, 10.18 mmol) prepared as anhydrous tributylammonium salt (prepared as described in Example 3 below) in 5 mL DMF and 2 mL tributylamine was added to the reaction in solution. The mixture was stirred at 50° C. for 3 days, then the solution was evaporated in vacuo into an oil, redissolved in 5 ml of water, and analyzed by column chromatography (300×50 mm, Sephadex DEAE-A25, 40-120 μ, Aldrich, pre-dissolved in 1.0 M ammonium bicarbonate Medium swe...
Embodiment 3A
[0057] Method for producing diuridine tetraphosphate with uridine 5'-triphosphate (UTP)
[0058] A 5 mL aqueous solution of uridine 5′-triphosphate (UTP) trisodium salt (ProBioSint, Varse, Italy; 5.86 g, 0.01 mol) was passed through a column of BioRad AG-MP 50 (Aldrich) strong cation exchange resin in the form of tributylamine (50 mL bed volume) and eluted with approximately 300 mL distilled water. Tributylamine (Aldrich, 5 mL) was added to this solution, and the suspension was shaken until the pH of the aqueous fraction rose to 8. The layers were separated and the aqueous solution was evaporated to a small volume and then lyophilized overnight. The residue was dissolved in dry dimethylformamide (Aldrich; 20 mL), and the solvent was evaporated at 0.1 mmHg. The dry tributylamine was made up to 100 mL with anhydrous acetone to obtain a stock solution (0.1 M in UTP). Dicyclohexylcarbodiimide was added to an aliquot of the aforementioned UTP solution (10 mL, 1.0 mmol), and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com