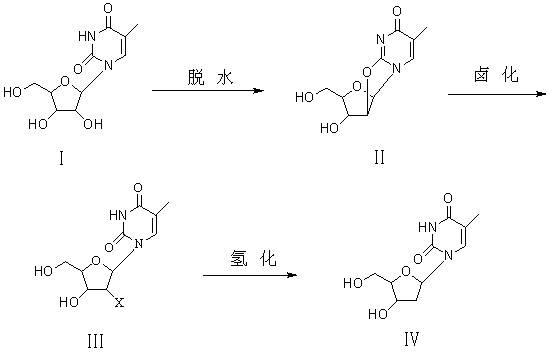
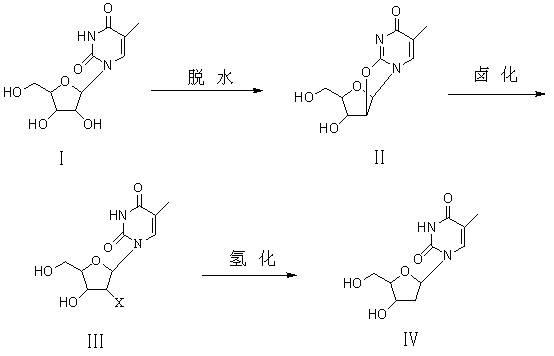
Preparation method of beta-thymidine
A technology of thymidine and equation, applied in the field of preparation of β-thymidine, can solve the problems of lack of stereospecificity of β-thymidine reaction, low utilization rate of raw materials, complicated separation process, etc., and achieve low cost, high cost and high product quality The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Add 25.8g of 5-methyluridine (0.1mol) and 25.7g of diphenyl carbonate (0.12mol) into 60ml of MDAC, start stirring and raise the temperature to 90°C, after the solid dissolves, add 1g of NaOH to reflux After keeping for 1 hour, after using TCL to measure the absence of raw materials, the solvent was distilled off under reduced pressure under cooling. After the distillation, cooling crystallized and filtered to obtain 21.9 g of intermediate (II) in the form of colorless crystal powder, with a yield of 94%.
[0027] In this embodiment, diphenyl carbonate is replaced with dimethyl carbonate, diethyl carbonate, dipropyl carbonate or dibutyl carbonate; the solvent is replaced with one or more mixed solvents in DMF, MDAC or acetonitrile MDAC, using KOH instead of NaOH, can achieve the above effects.
Embodiment 2
[0028] Example 2 Add 25.8g of 5-methyluridine (0.1mol) and 32.1g of diphenyl carbonate (0.15mol) into 60ml of DMF, start stirring and raise the temperature to 90°C, after the solid dissolves, add 1.5g of KOH to reflux After heat preservation for 2 hours, after no raw material was determined by TCL, the solvent was distilled off under reduced pressure under cooling, crystallized by cooling after distillation, and filtered to obtain 22.1 g of intermediate (II) in the form of colorless crystal powder, with a yield of 95%.
Embodiment 3
[0029] Example 3 Add 25.8g of 5-methyluridine (0.1mol) and 10.8g of dimethyl carbonate (0.12mol) into 50ml of DMF, start stirring and raise the temperature to 120°C, after the solid dissolves, add 1g of KOH to keep it under reflux After 3 hours, after no raw materials were measured with TCL, the solvent was distilled off under reduced pressure under cooling. After the distillation, cooling crystallized and filtered to obtain 22.1 g of colorless crystal powdery intermediate (II), with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com