Preparation method for sofosbuvir
A technology of sofosbuvir and uridine, which is applied in the field of preparation of drug sofosbuvir, can solve the problems of low conversion rate and many reaction steps, and achieve the effects of promoting development, easy availability of raw materials, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
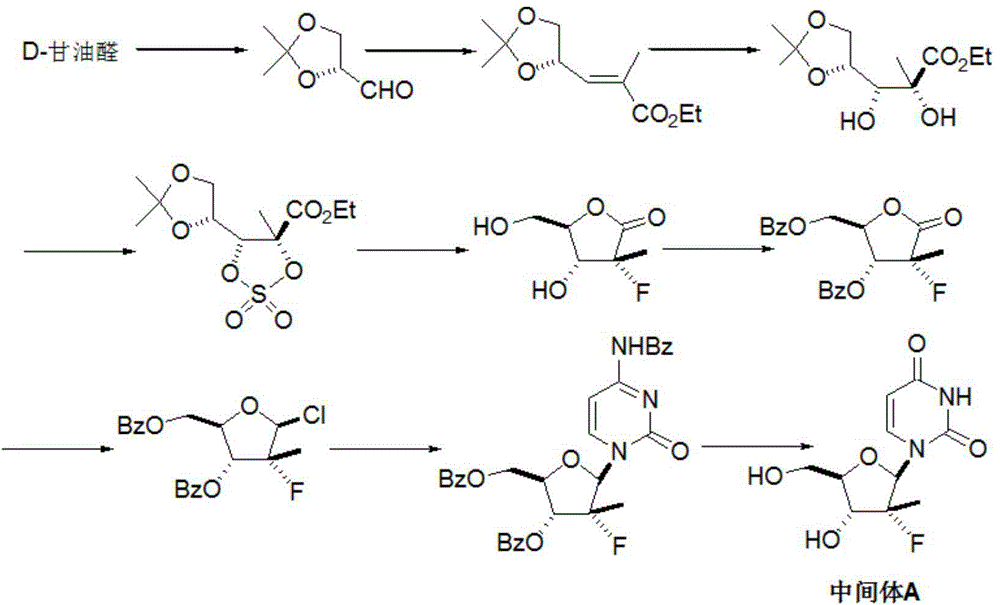
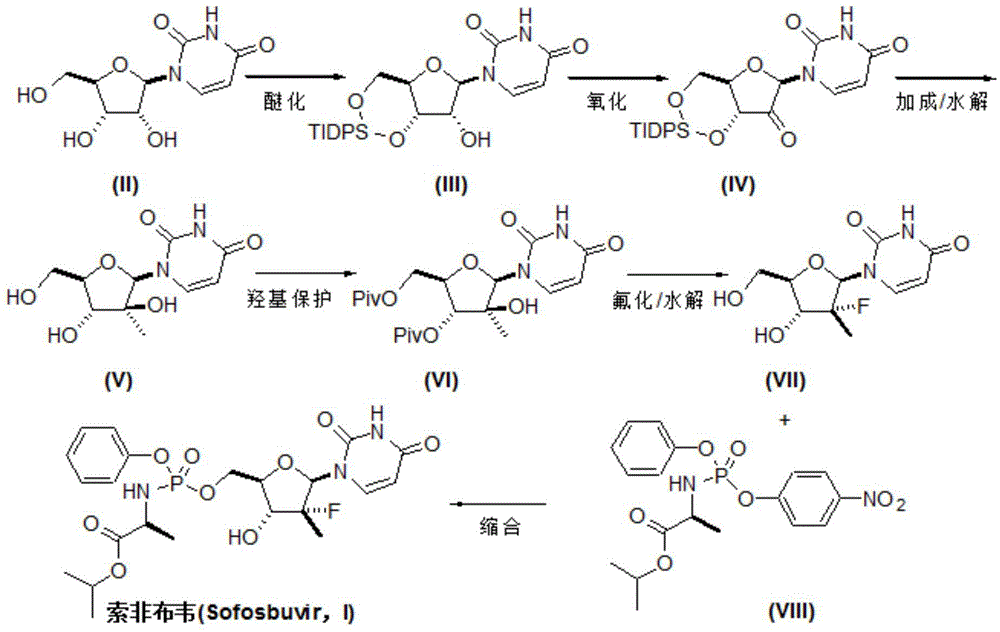
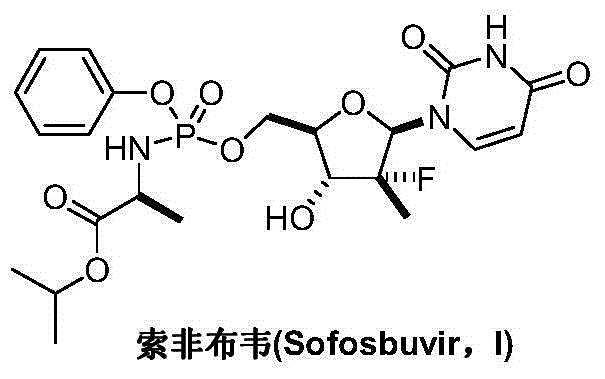
[0029] Add uridine (II) (12.2g, 50mmol) and 250mL of anhydrous pyridine into the reaction flask, add 1,3-dichloro-1,1,3,3- Tetraisopropyl-1,3-disiloxane (16.0 mL, 50 mmol) was added after dropping, warmed up to room temperature, and reacted for 12 hours, and the reaction was detected by TLC. Cool in an ice bath and quench the reaction with 5 mL of water. The solvent was recovered under reduced pressure, the residue was dissolved in dichloromethane, washed with water, saturated brine and water successively, dried over anhydrous sodium sulfate, concentrated, and the obtained crude product was weighed with dichloromethane and n-hexane (4:1, V / V). Crystallized and dried in vacuo to obtain 16.8 g of white solid 3',5'-O-(tetraisopropyldisiloxane-1,3-diether)uridine (III), yield 77.1%, mass spectrum (EI): m / z 437 (M+H).
Embodiment 2
[0031] Add 3',5'-O-(tetraisopropyldisiloxane-1,3-diether)uridine (III) (8.74g, 20mmol) into the reaction flask, Molecular sieves (5 g) and dichloromethane 100 mL were added to PCC (10.8 g, 50 mmol) in batches at room temperature, and reacted at room temperature for 14 hours, and the reaction was detected by TLC. Add 200 mL of diethyl ether, stir and filter, and wash the filter cake twice with diethyl ether. The organic phases were combined, and the resulting residue was purified by silica gel column (diethyl ether / n-hexane: 1 / 1), concentrated, and dried in vacuo to obtain a white solid 2'-carbonyl-3',5'-O-(tetraisopropyldi Siloxane-1,3-diether)uridine (IV) 7.47g, yield 85.9%, mass spectrum (EI): m / z 435 (M+H).
Embodiment 3
[0033] Add 3',5'-O-(tetraisopropyldisiloxane-1,3-diether)uridine (III) (8.74g, 20mmol) and 100mL tetrahydrofuran into the reaction flask, add DMSO ( 4.7g, 60mmol), the temperature was lowered to -78°C, and a solution of oxalyl chloride (3.8g, 30mmol) in anhydrous tetrahydrofuran (50mL) was added dropwise. After about 3 hours of dripping, the temperature was slowly raised to 0° C., and the reaction was continued for 2 hours, and the reaction was detected by TLC. Triethylamine was added to neutralize excess acid. The solvent was distilled off under reduced pressure, and the residue was purified by silica gel column (diethyl ether / n-hexane 1 / 1), concentrated, and dried in vacuo to obtain a white solid 2'-carbonyl-3',5'-O-(tetraisopropyldisilazol Oxyalkane-1,3-diether)uridine (IV) 7.85g, yield 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com