Butylphthalide nasal drop and preparation method thereof
A technology of butylphthalide and nasal drops, applied in the field of medicine, can solve problems such as undisclosed technical solutions of butylphthalide nasal drops, and achieve the effects of small size, fast onset of action and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
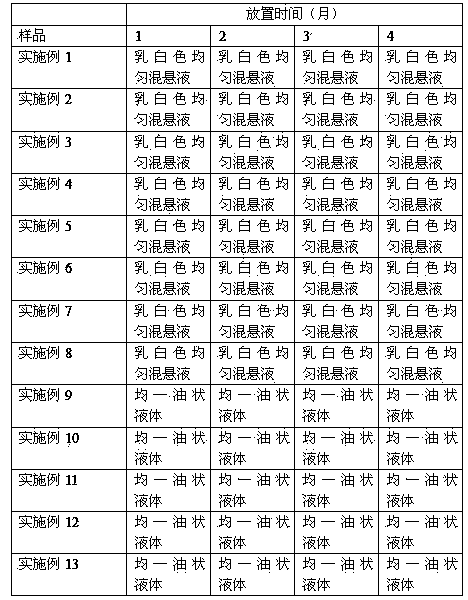
Examples
Embodiment 1
[0044] Prescription composition:
[0045] components Dosage ( g ) Weight percentage (%) rac-Butylphthalide 1.5 7.89 medium chain glycerides 1.5 7.89 Water for Injection 10.0 52.63 Polyoxyethylene 40 Hydrogenated Castor Oil 3.0 15.79 Vitamin E 1.0 5.26 Mannitol 1.0 5.26 citric acid 1.0 5.26
[0046] Preparation:
[0047] (1) Prepare the oil phase: weigh 1.5 g of butylphthalide and 1.5 g of medium-chain glyceride, mix well, and preheat in a 60°C water bath;
[0048] (2) Prepare the water phase: weigh 1.0g of mannitol\3.0g of polyoxyethylene 40 hydrogenated castor oil, 1.0g of vitamin E and 1.0 of mannitol in 10.0g of water for injection, and preheat in a water bath at 60°C;
[0049] (3) Preparation of colostrum: Slowly pour the oil phase obtained in step (1) into the water phase obtained in step (2), and disperse with a high-speed shear dispersing emulsifier for 5 minutes at 10,000 rpm. Colostrum is prepared by ...
Embodiment 2
[0054] prescription composition
[0055] components Dosage ( g ) Weight percentage (%) rac-Butylphthalide 3.0 16.67 Water for Injection 10.0 55.56 Polysorbate 80 1.5 8.33 Oleic acid 1.5 8.33 Sodium chloride 1.5 8.33 citric acid 0.5 2.78
[0056] Preparation:
[0057] (1) Prepare the oil phase: weigh 3.0 g of butylphthalide and preheat in a water bath at 60°C;
[0058] (2) Prepare the water phase: weigh 1.5g of polysorbate 80, 1.5g of sodium chloride and oleic acid in 10.0g of 15g of water for injection, and preheat in a water bath at 60°C;
[0059] (3) Preparation of colostrum: Slowly pour the oil phase obtained in step (1) into the water phase obtained in step (2), and disperse with a high-speed shear dispersing emulsifier for 5 minutes at 10,000 rpm. Colostrum is prepared by ultrasonic method or high-speed shear Method (FA25 laboratory high-shear dispersing emulsifier, Shanghai Fluke Electromechanical Equipment Co...
Embodiment 3
[0064] Prescription composition:
[0065] components Dosage ( g ) Weight percentage (%) rac-Butylphthalide 4.5 25.00 Water for Injection 10.0 55.56 Polyoxyethylene 35 castor oil 1.5 8.33 Mannitol 1.0 5.56 citric acid 1.0 5.56
[0066] Preparation:
[0067] (1) Prepare the oil phase: weigh 4.5 g of butylphthalide, and preheat it in a water bath at 60°C;
[0068] (2) Prepare the water phase: weigh 1.5g of polyoxyethylene 35 castor oil and 1.0g of mannitol into 10.0g of water for injection, and preheat in a 60°C water bath;
[0069] (3) Preparation of colostrum: Slowly pour the oil phase obtained in step (1) into the water phase obtained in step (2), and disperse with a high-speed shear dispersing emulsifier for 5 minutes at 10,000 rpm. Colostrum is prepared by ultrasonic method or high-speed shear Method (FA25 laboratory high-shear dispersing emulsifier, Shanghai Fluke Electromechanical Equipment Co., Ltd.);
[0070] (4) Ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com