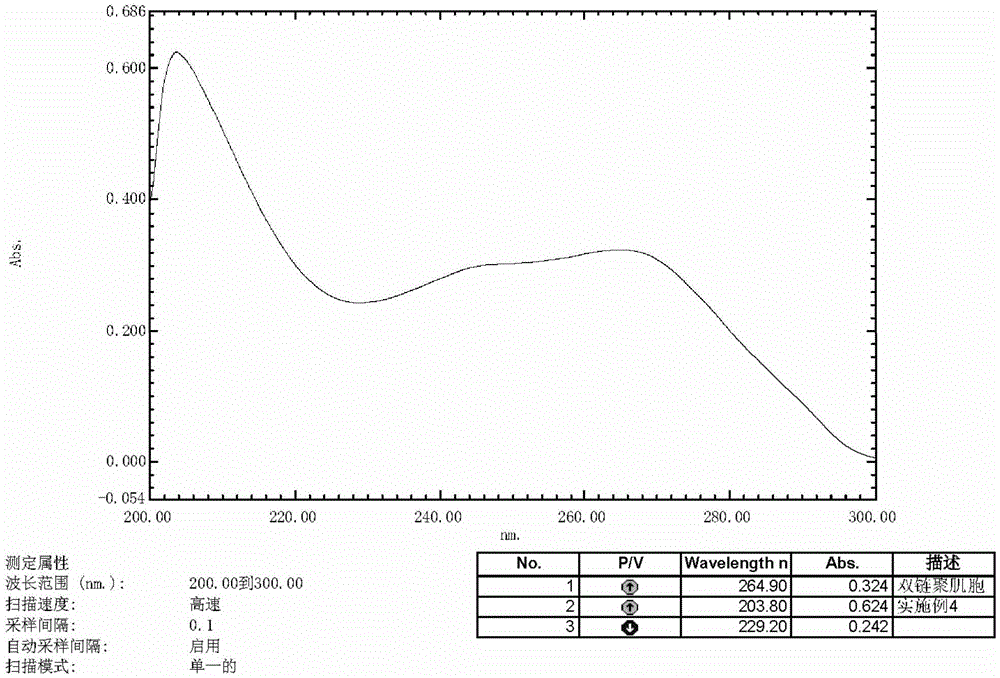
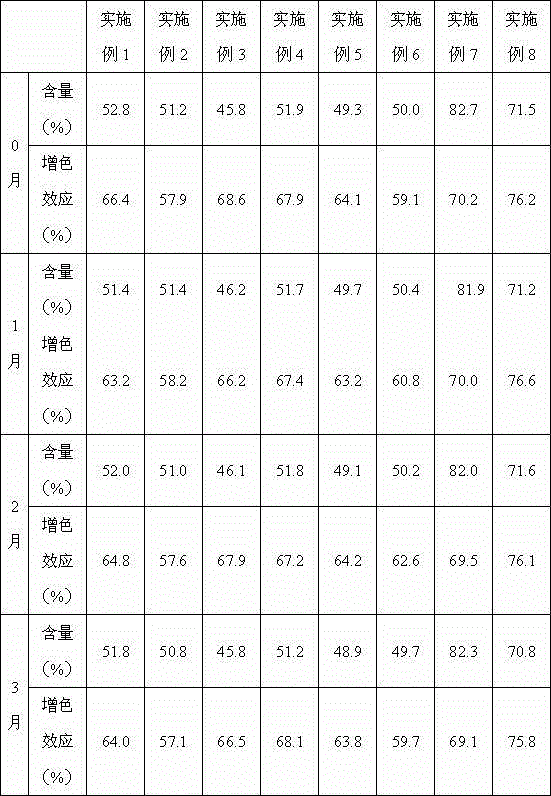
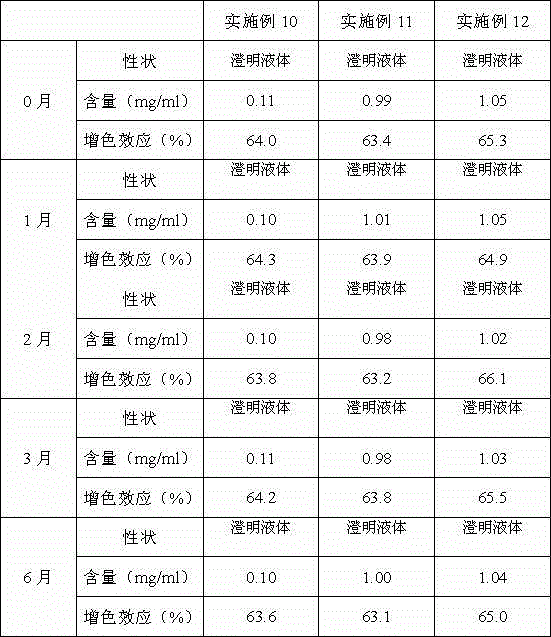
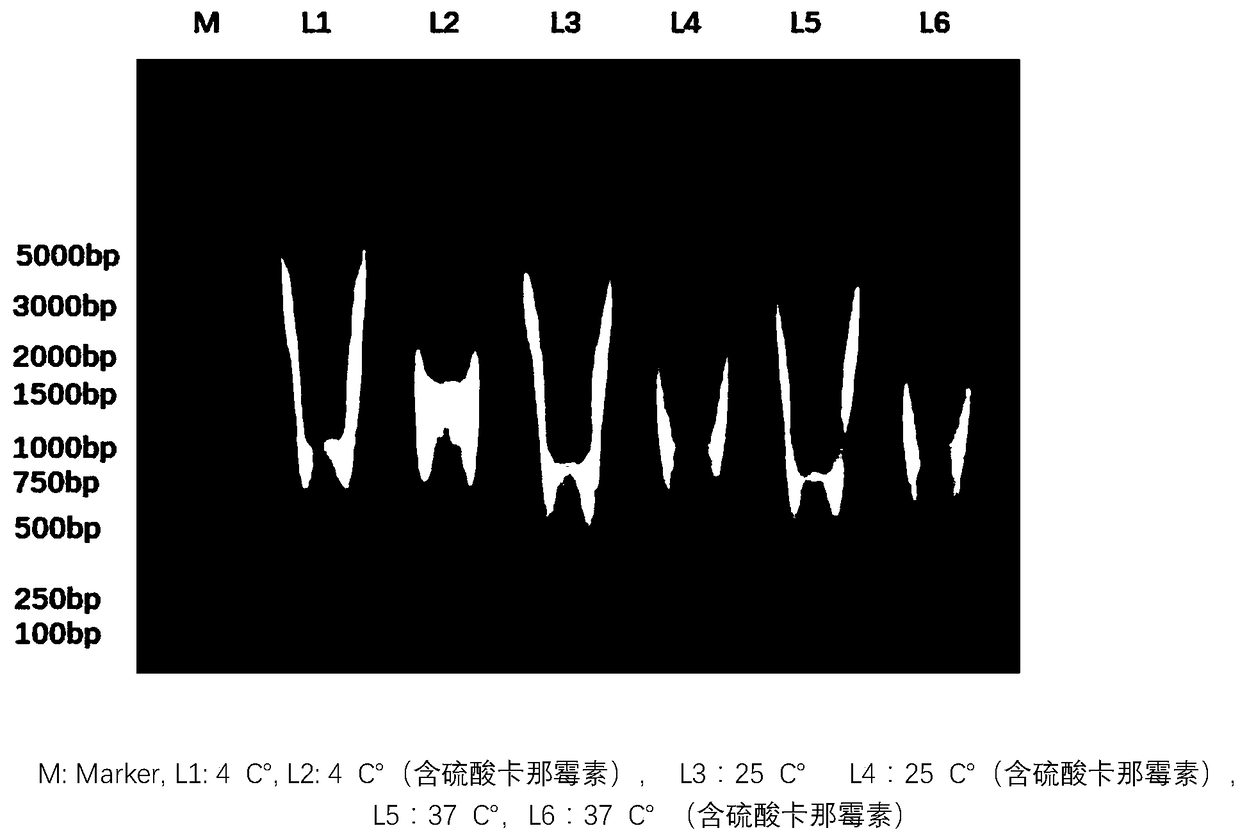
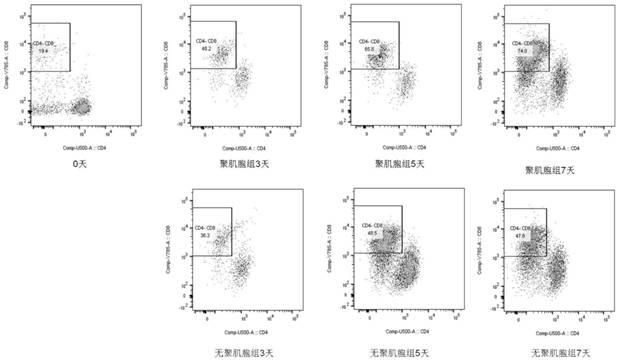
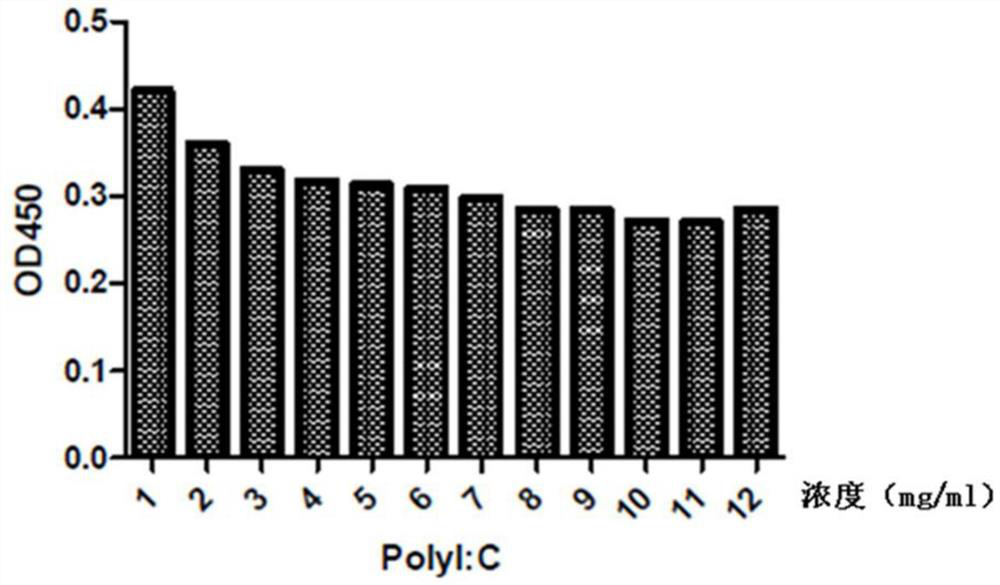
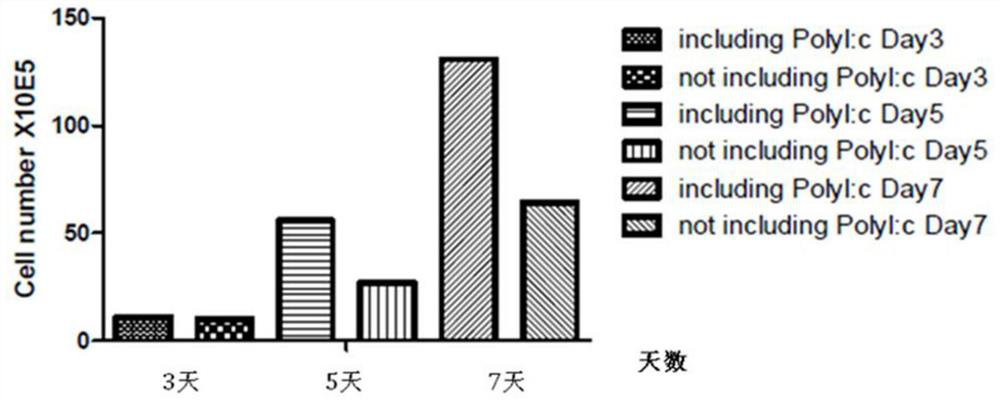
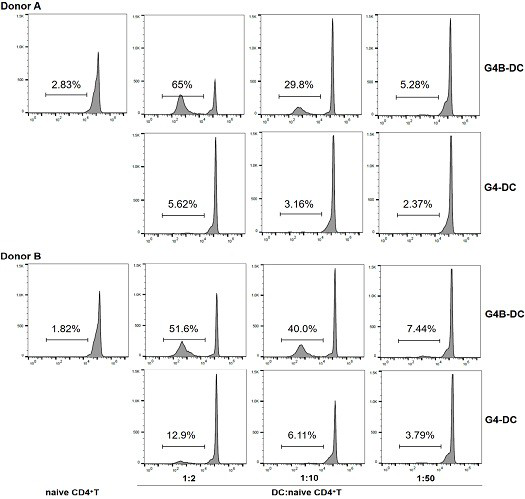
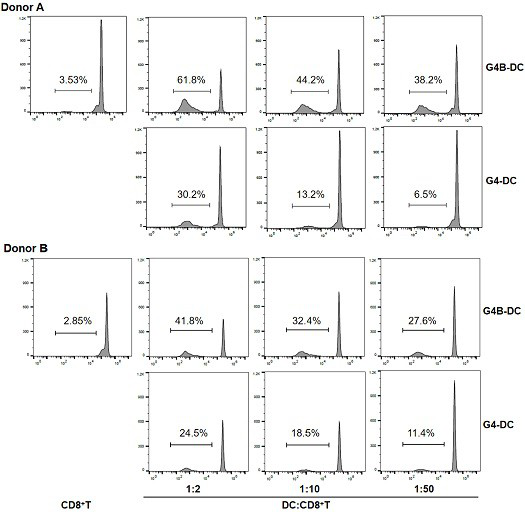
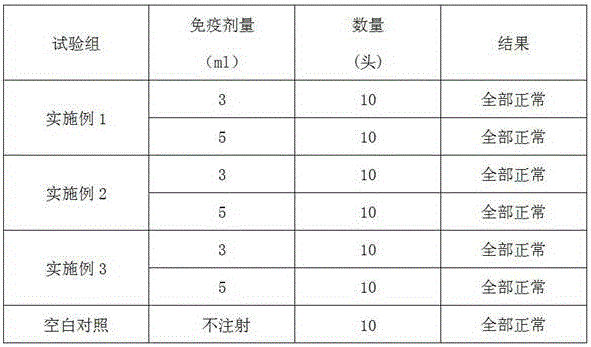
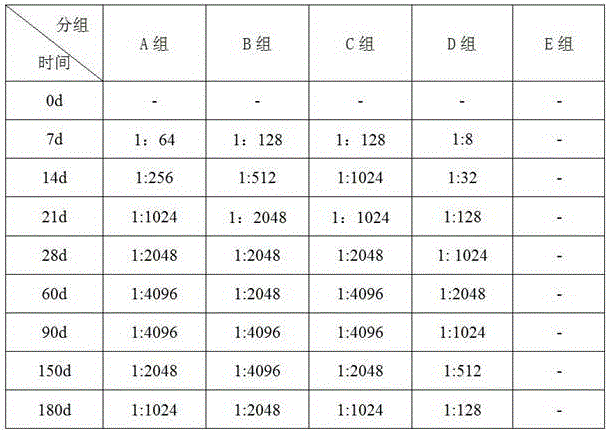
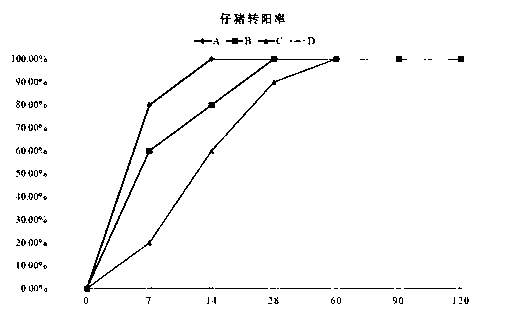
Patents
Literature
40 results about "Polyinosinic:polycytidylic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyinosinic:polycytidylic acid (usually abbreviated poly I:C or poly(I:C)) is an immunostimulant. It is used in the form of its sodium salt to simulate viral infections. Poly I:C is known to interact with toll-like receptor 3 (TLR3), which is expressed at the endosomal membrane of B-cells, macrophages and dendritic cells. Poly I:C is structurally similar to double-stranded RNA, which is present in some viruses and is a "natural" stimulant of TLR3. Thus, Poly I:C can be considered a synthetic analog of double-stranded RNA and is a common tool for scientific research on the immune system.
Preparation method of polyinosinic-polycytidylic acid dry powder
ActiveCN103599071AEasy to makeEasy to operatePowder deliveryCosmetic preparationsBiological bodyOrganic solvent
The invention provides a preparation method of polyinosinic-polycytidylic acid dry powder. The method comprises the following steps: (1) respectively dissolving polyinosinic acid and polycytidysic acid in a phosphate buffer solution, mixing the solution, adding a stabilizer, uniformly mixing the stabilizer and the solution, and performing heat preservation on the mixture at the temperature of 40-100DEG C; (2) naturally cooling the reaction liquid obtained in the step (1), mixing the reaction liquid with an organic solvent, standing for precipitating, and drying the precipitate, thereby obtaining the polyinosinic-polycytidylic acid dry powder. The preparation method has the beneficial effects that the prepared polyinosinic-polycytidylic acid dry powder has a complete double-helix structural features and physiological activity, the quality and the stability are better than those of the polyinosinic-polycytidylic acid at a solution state, a stronger enzymolysis-resisting performance can be achieved compared with the bare polyinosinic-polycytidylic acid after entering a living body, so that the curative effect can be enhanced. The polyinosinic-polycytidylic acid is purified in the process for making the polyinosinic-polycytidylic acid solution into the dry powder, a plurality of small-molecular-weight substances and impurities can be removed, the toxic and side effects can be reduced, and the quality of the polyinosinic-polycytidylic acid can be improved.
Owner:美亚药业海安有限公司
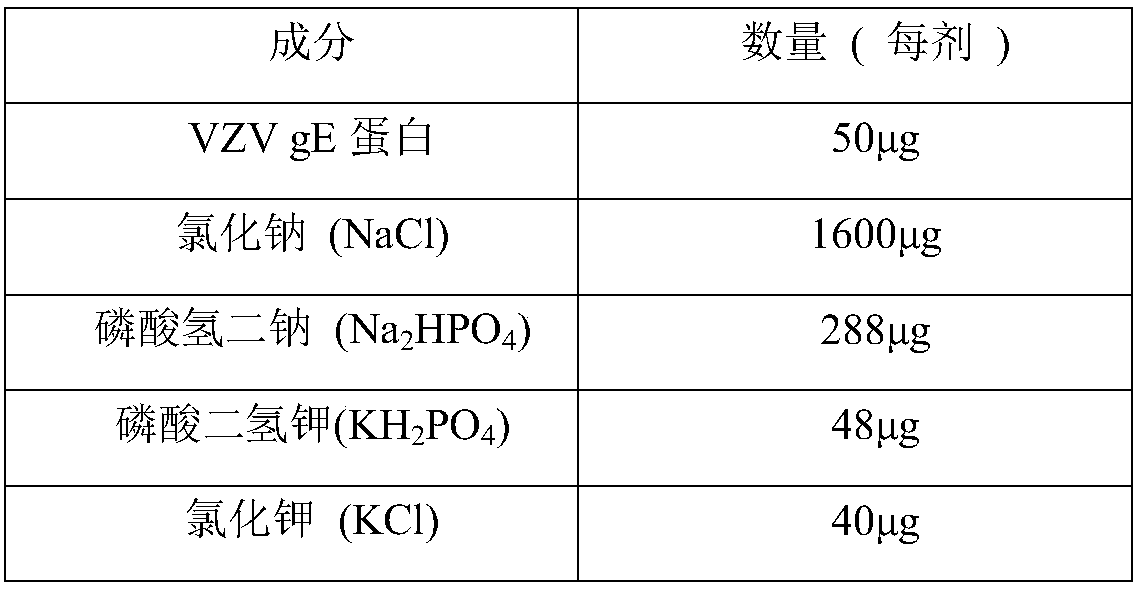
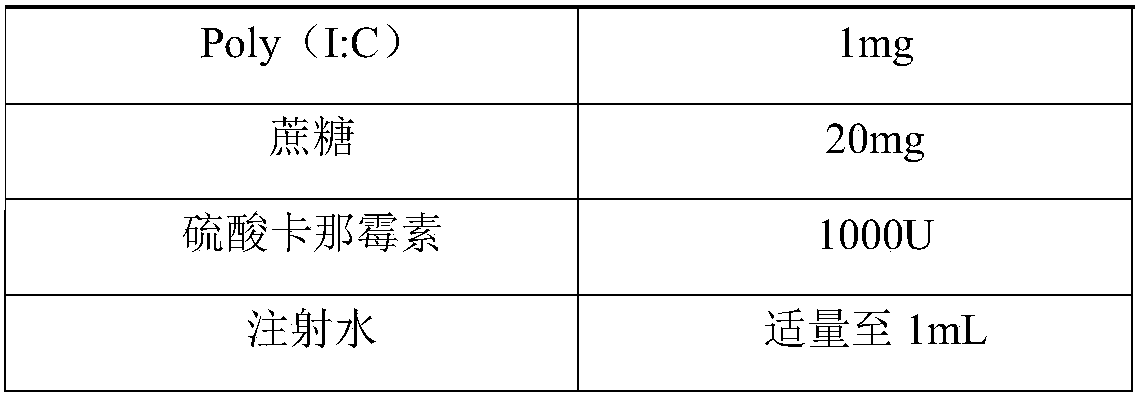
Herpes zoster vaccine, and preparation method and application thereof
InactiveCN108992667AImprove immunityEnhance humoral immune responseNervous disorderViral antigen ingredientsAdjuvantIn vivo
The invention discloses a herpes zoster vaccine, and a preparation method and an application thereof, and belongs to the field of vaccines. The herpes zoster vaccine is provided against the problem ofhigh safety risk of existing herpes zoster vaccines, and an adjuvant used by the herpes zoster vaccine is Poly (I:C). The Poly(I:C) is called polyinosinic-polycytidylic acid for short, and is double-stranded RNA, and the safety of the Poly(I:C) is confirmed during large-scale clinical application. The Poly(I:C) is a high-efficiency interferon inducer, can produce an immune reaction like viral infection in vivo, and can induce CD4<+> and CD8<+> T cells and enhance cellular and humoral immune responses.
Owner:ANHUI ZHIFEI LONGCOM BIOPHARM CO LTD +2
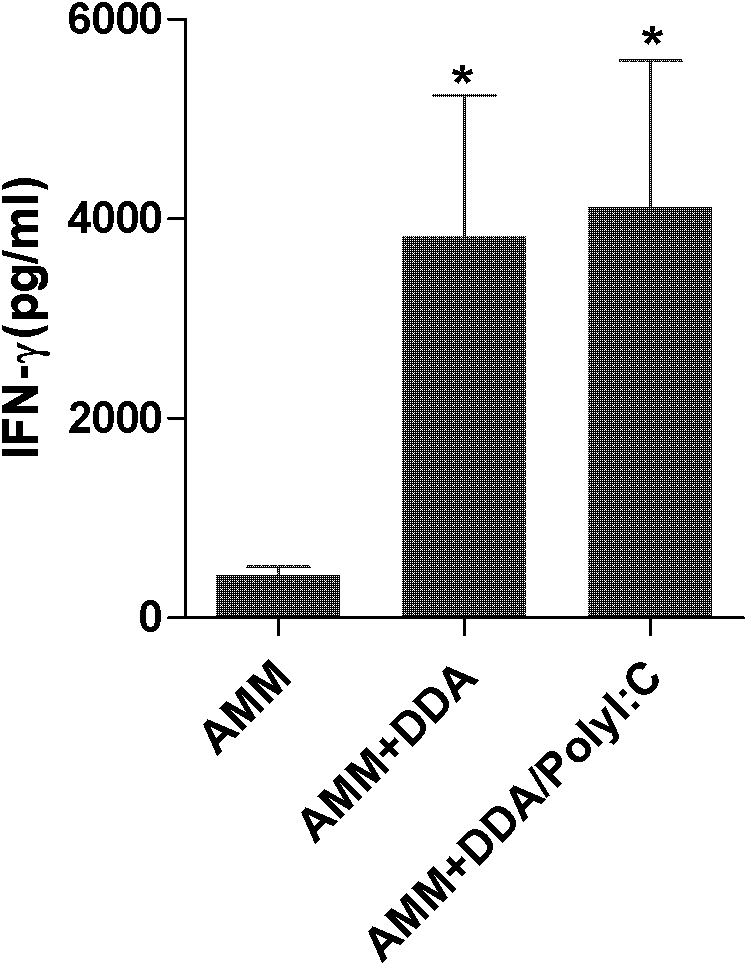
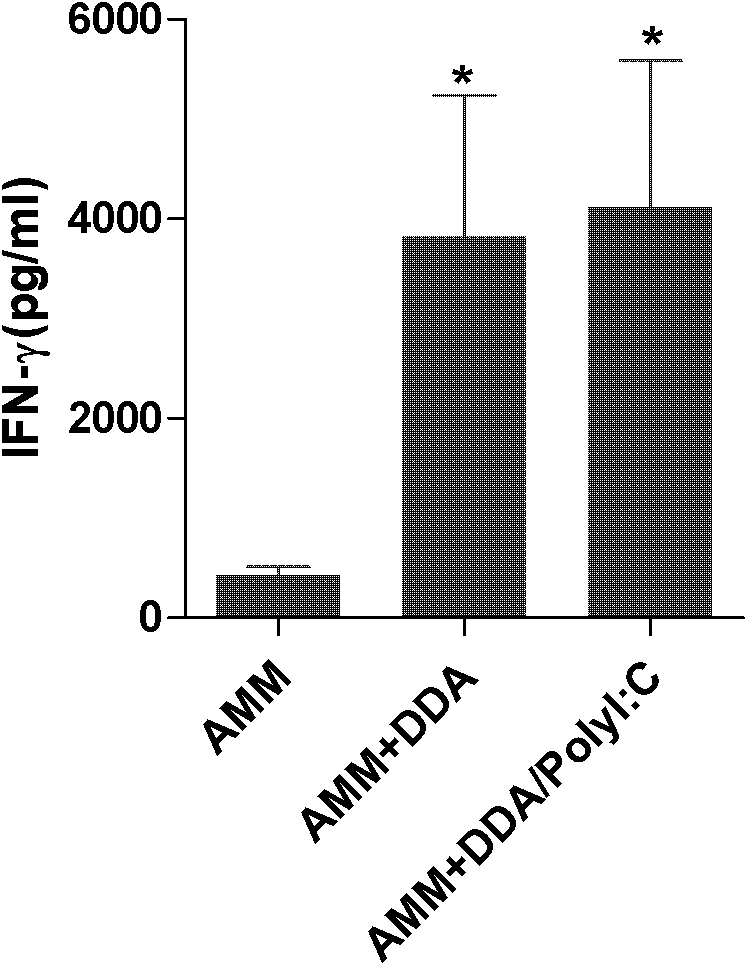
Application of polyinosinic-polycytidylic acid (poly I:C) combined dimo-thylidioctyl ammonium bromide (DDA) mixed adjuvant to preparation of tuberculosis subunit vaccines
ActiveCN102247595AIncrease secretion levelReduce pathological damageAntibacterial agentsBacterial antigen ingredientsVaccination against tuberculosisAdjuvant
The invention discloses application of a polyinosinic-polycytidylic acid (poly I:C) combined dimo-thylidioctyl ammonium bromide (DDA) mixed adjuvant to the preparation of tuberculosis subunit vaccines. The invention has the advantages that: the mixed adjuvant DDA / Poly I:C can enhance the secretion level of antigenic specificity interferon-gamma (IFN-gamma) of fusion protein Ag85B-MPT64190-198-Mtb8.4 (AMM) effectively and relieve pathological damage caused by H37Rv infection, and is an effective adjuvant of vaccines for preventing tuberculosis.
Owner:LANZHOU UNIVERSITY
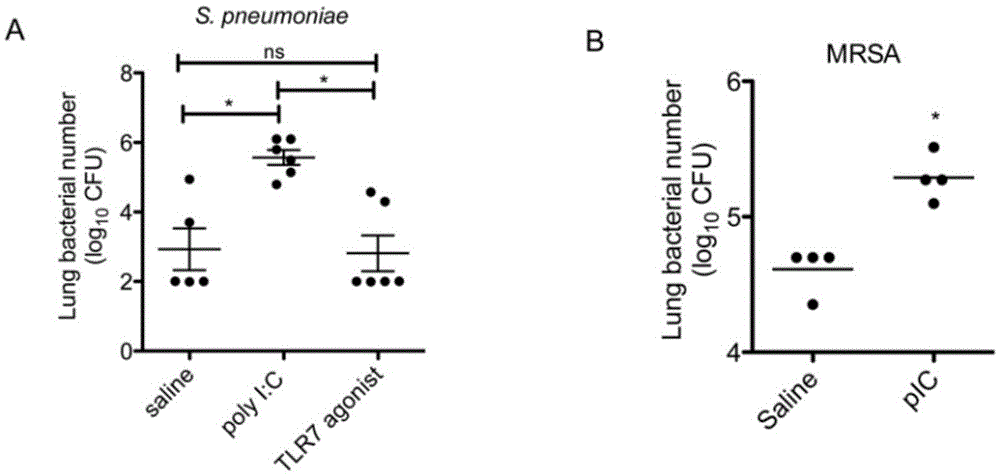
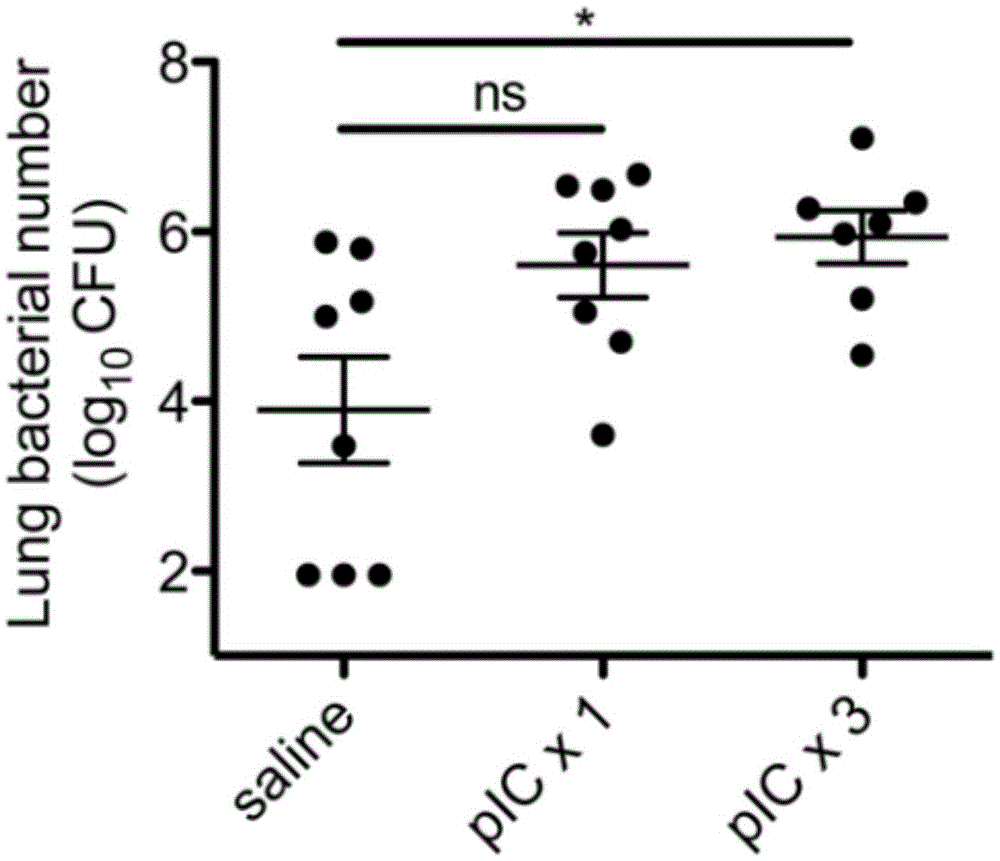
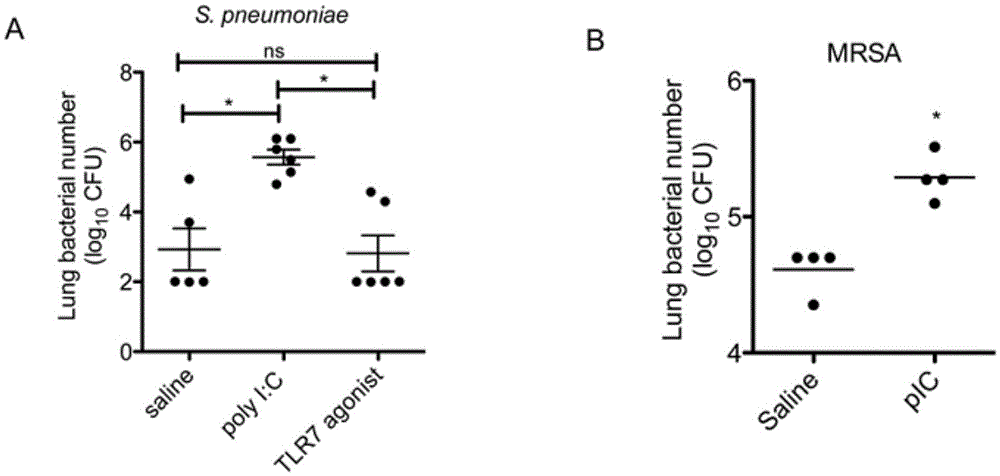
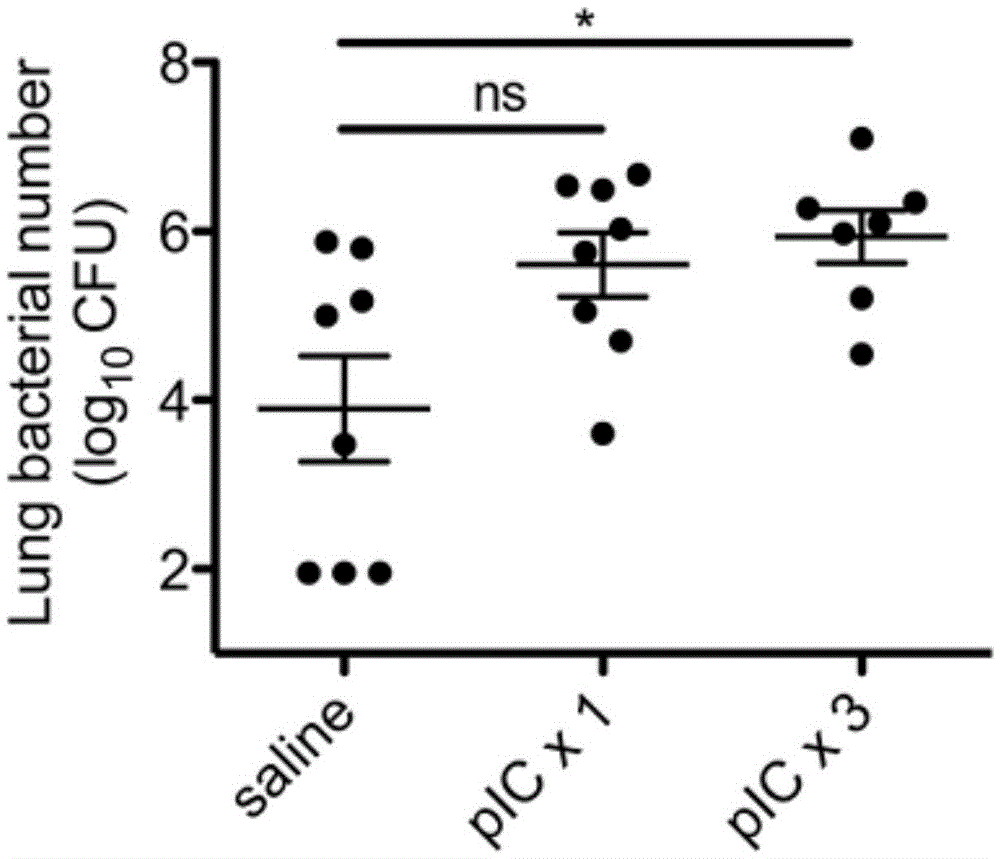
Application of poly inosine-cytidine, imiquimod and gardiquimod in constructing virus immunity mouse model
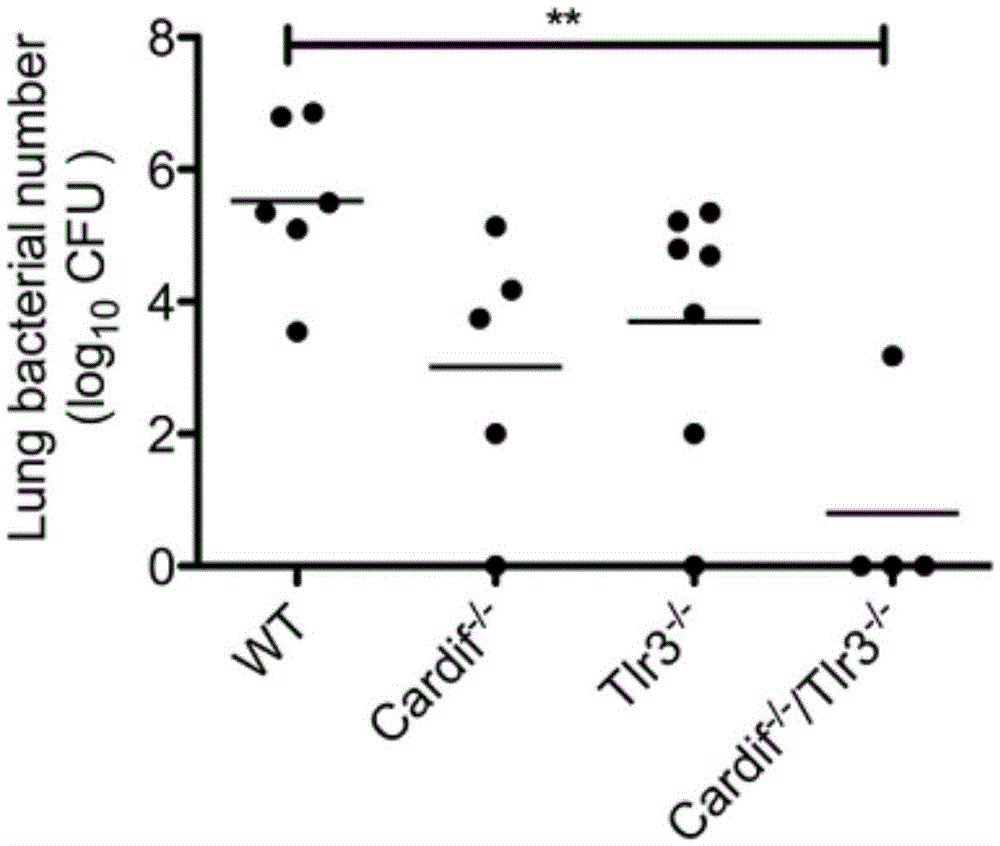
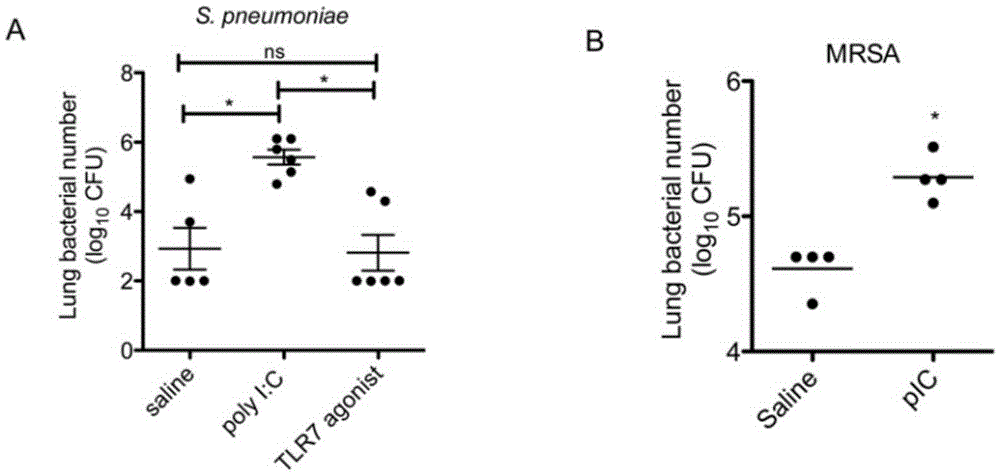
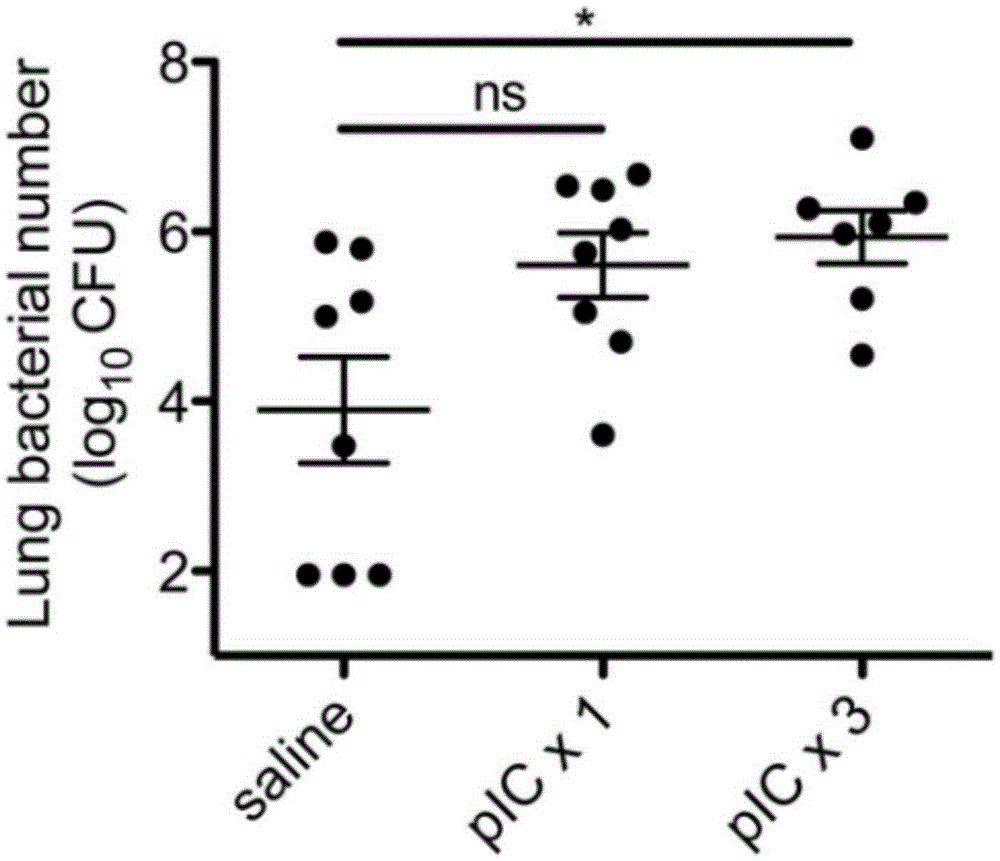
The invention relates to the field of biological medicine, in particular to an application of poly inosine-cytidine, imiquimod and gardiquimod in constructing a virus immunity mouse model. The inventor of the invention employs the mouse model and forms an antivirus immune state by using poly inosine-cytidine, imiquimod and gardiquimod; an experiment mouse administered with poly inosine-cytidine is found to appear lung bacterium clearance impairment; in addition, poly inosine-cytidine is found to be capable of mediating IFNI expression; and finally, IFNI can induce a defense mechanism of a lung against gram positive pathogenic bacteria.
Owner:宁波美丽人生医药生物科技发展有限公司
Method for efficiently producing DC-CIK cells through induction of polyinosinic: polycytidylic acid copolymer
PendingCN111733129AEnhance tumor killing activityEasy to killCulture processBlood/immune system cellsCancer cellSpecific immunity
The invention relates to the technical field of immunization, in particular to a polyinosinic: polycytidylic acid copolymer (Poly I: C). The polyinosinic: polycytidylic acid copolymer is a ligand of atype-3 Toll-like receptor in an animal body, can mediate a series of immune reactions of the body after TLR-3 is activated, and has a good promoting effect on specific immunity and non-specific immunity of the body. Compared with a control group, DC-CIK induced by the polyinosinic: polycytidylic acid copolymer has higher proliferation, high differentiation (especially CD3+CD4-CD8+, CTL) and hightumor killing activity. The autologous efficient DC-CIK induced by the polyinosinic: polycytidylic acid copolymer and other immune cells are applied to treatment of clinical tumor patients. The polyinosinic: polycytidylic acid copolymer aims to recover or improve the immune function of the tumor patients, and the immune system of the body is improved to kill and inhibit proliferation of tumor cells. The tumor cell load is reduced, tiny residual lesions are removed, or the proliferation mode of residual tumor / cancer cells is obviously inhibited, factors such as relapse and metastasis are eliminated, the cure possibility is increased, the survival time is prolonged, and the life quality is improved. Therefore, the purpose of treating tumors / cancers is achieved.
Owner:成都源泉生物科技有限公司 +1
Application of polyinosinic-polycytidylic acid (poly I:C) combined dimo-thylidioctyl ammonium bromide (DDA) mixed adjuvant in preparation of tuberculosis subunit vaccines
ActiveCN102247595BIncrease secretion levelReduce pathological damageAntibacterial agentsBacterial antigen ingredientsVaccination against tuberculosisAdjuvant
The invention discloses application of a polyinosinic-polycytidylic acid (poly I:C) combined dimo-thylidioctyl ammonium bromide (DDA) mixed adjuvant to the preparation of tuberculosis subunit vaccines. The invention has the advantages that: the mixed adjuvant DDA / Poly I:C can enhance the secretion level of antigenic specificity interferon-gamma (IFN-gamma) of fusion protein Ag85B-MPT64190-198-Mtb8.4 (AMM) effectively and relieve pathological damage caused by H37Rv infection, and is an effective adjuvant of vaccines for preventing tuberculosis.
Owner:LANZHOU UNIVERSITY
Anti-infection and Anti-tumor mucosal immune preparation
ActiveUS20180360738A1Certain activityCertain characteristicAntibacterial agentsPowder deliveryChemical LinkageAbnormal tissue growth
The present invention relates to an anti-infection and anti-tumor mucosal immune preparation. The mucosal immune preparation includes mucosal immune substances that are mainly formed by organically bonding polyinosinic-polycytidylic acid, non-antibiotic amino compounds and metal cations through chemical bonds. The present invention provides a slow release effect on a local part or the whole body, prevents the degradation of serum ribonucleases of human beings and primates, prolong the half-life period of the mucosal immune preparation, increases the availability and effectiveness of drugs. The mucosal immune preparation can facilitate the mucosal immunity of the body by mucosal immunity and thus facilitate the activation and proliferation of various immune cells, rather than merely acting on diseased local parts, so that the purposes of anti-infection and anti-tumor prevention and treatment with almost no side effects are realized. Mucosal immunity also avoids the pain of repeated injection so that good compliance is achieved.
Owner:LIN HAIXIANG +3
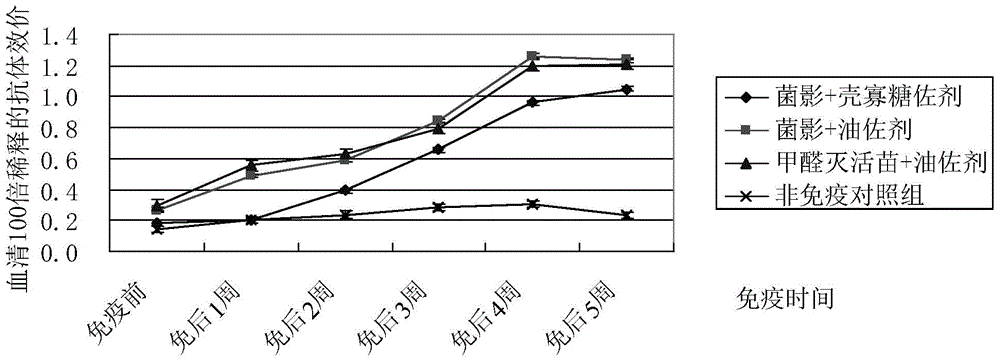
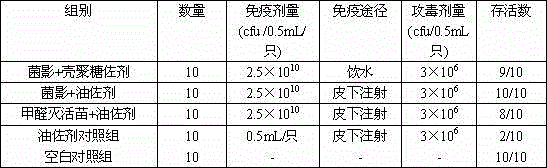
Riemerella anatipestifer bacterial ghost vaccine adopting chitosan oligosaccharide as adjuvant
InactiveCN104971346AImprove the level ofAvoid the stress of immunizationAntibacterial agentsBacterial antigen ingredientsPenicillinAdjuvant
The purpose of the present invention is to provide a riemerella anatipestifer bacterial ghost vaccine loaded with polyinosinic acid-polycytidylic acid having interferon inducing activity and added with chitosan oligosaccharide as an adjuvant. According to the technical scheme, a temperature control expression vector is constructed and is electrotransformed into riemerella anatipestifer protoplast, positive clones are screened and then are subjected to bacterial amplification culture at a temperature of 28 DEG C, lysis gene expression is induced at a temperature of 42 DEG C, polylysine is added to continuously act when the OD600 is no longer be reduced so as to completely lyze the live bacteria, centrifugation is performed to separate the bacteria, the dried bacteria and a 6 mg / mL Poly I:C solution according to an equal ratio so as to make the Poly I:C be embedded into the bacterial ghosts, a 2% chitosan oligosaccharide aqueous solution is added, stirring is performed to form a uniform mixture, sub-packaging is performed into penicillin bottles, and freeze-drying is performed so as to obtain the novel riemerella anatipestifer bacterial ghost vaccine. According to the present invention, the prepared riemerella anatipestifer bacterial ghost vaccine is immunized through drinking water, such that the local immunity on the respiratory tract mucous membrane and the digestive tract mucous membrane can be irritated, the humoral immunity can be stimulated, and the protection effect on the human body is not different from the injection immunity.
Owner:QINGDAO AGRI UNIV
Human dendritic cell induction method and composition for resisting viruses and tumors
ActiveCN113444688AFunctionalShort training timeCulture processAntiviralsDendritic cellWhite blood cell
The invention relates to the field of cell culture and virus and tumor resistance of immune cells, and particularly discloses a human dendritic cell induction method and composition for virus and tumor resistance. The human dendritic cell induction composition comprises a mature human dendritic cell induction composition and an immature human dendritic cell induction composition, wherein the mature human dendritic cell induction composition consists of poly I: C and recombinant human interleukin 1 beta (IL-1beta), and the immature human dendritic cell induction composition consists of a granulocyte-macrophage colony stimulating factor (GM-CSF), recombinant human interleukin 4 (IL-4) and interferon beta (IFN-beta). The human dendritic cell induction method based on the human dendritic cell induction composition is used for inducing and culturing for 3 days to obtain a DC which is short in culture period, strong in aggregation, strong in function of inducing allogenic initial CD4 + T cells and CD8 + T cells and capable of inducing antigen-specific CD8 + T cells; the culture period can be greatly shortened by utilizing the method, and the highly-functional DC is obtained and is used as an early-stage basis of virus resistance and tumor resistance of clinical cell vaccines.
Owner:GUANGXI UNIV OF CHINESE MEDICINE
Diluent used for inactive porcine parvovirus vaccine
ActiveCN106727985AEnhance immune functionActivate immune functionOrganic active ingredientsViral antigen ingredientsDiluentLycium barbarum fruit
The invention belongs to the technical field of veterinary biological products, and concretely relates to a diluent used for an inactive porcine parvovirus vaccine. Every 1000 ml of a phosphate buffer solution with the pH value of 7 includes 1-5 g of honeysuckle flower and Weeping Forsythia extract, 5-10 g of wolfberry fruit polysaccharides, 20-80 mg of astragaloside II and 1-5 g of polyinosinic-polycytidylic acid. The diluent used for the inactive porcine parvovirus vaccine has the advantages of convenience in production, low cost, convenience and safety in use, reduction of the difference among different batches of vaccines, and improvement of the integral immunizing effect of the vaccine.
Owner:浙江美保龙生物技术有限公司
Polyinosinic-polycytidylic acid injection and method for lowering toxins in polyinosinic-polycytidylic acid injection
PendingCN110433173AReduce contentControl contentOrganic active ingredientsSenses disorderPolymer solutionToxin
Owner:CHENGDU HAITONG PHARMA
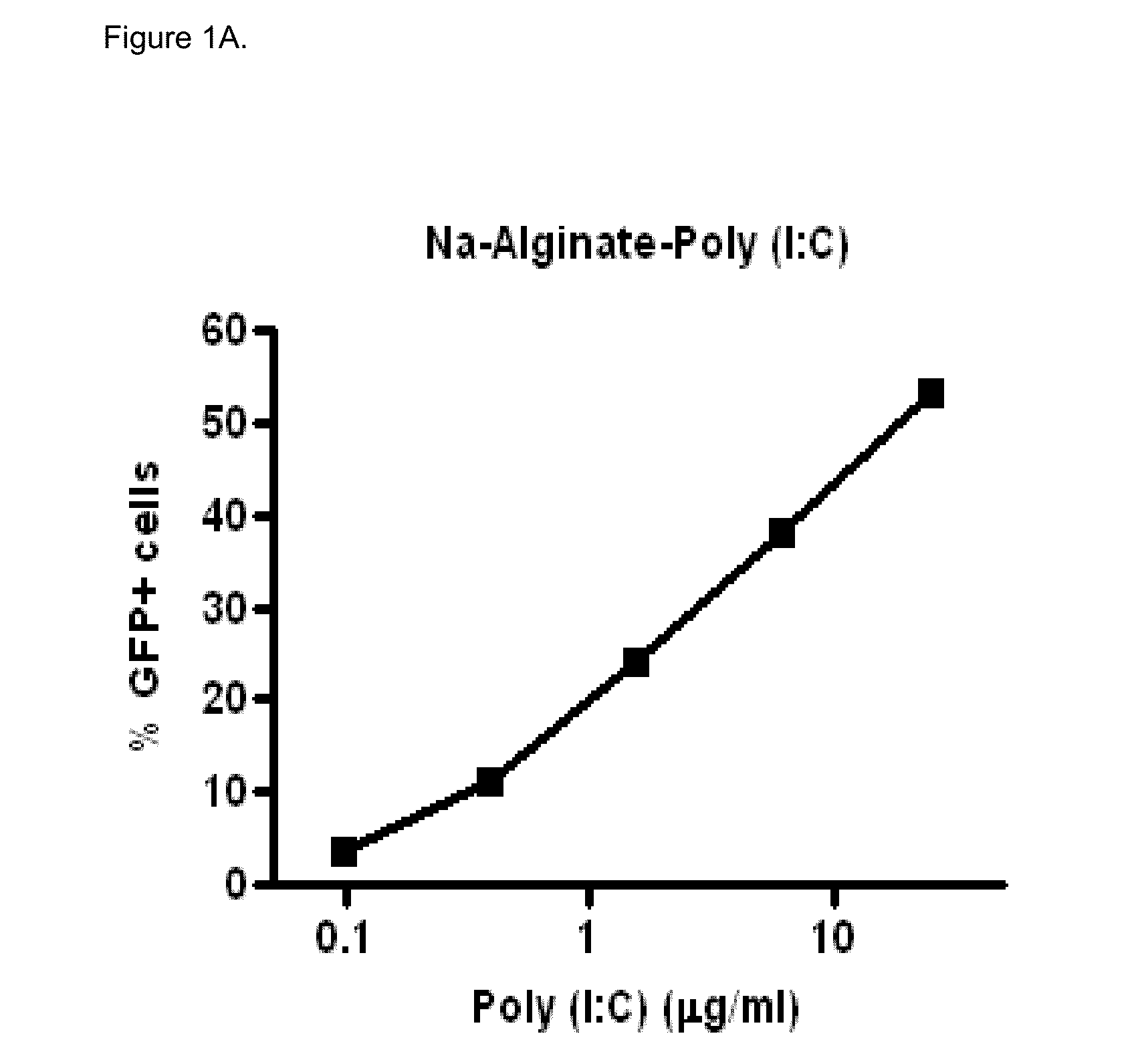
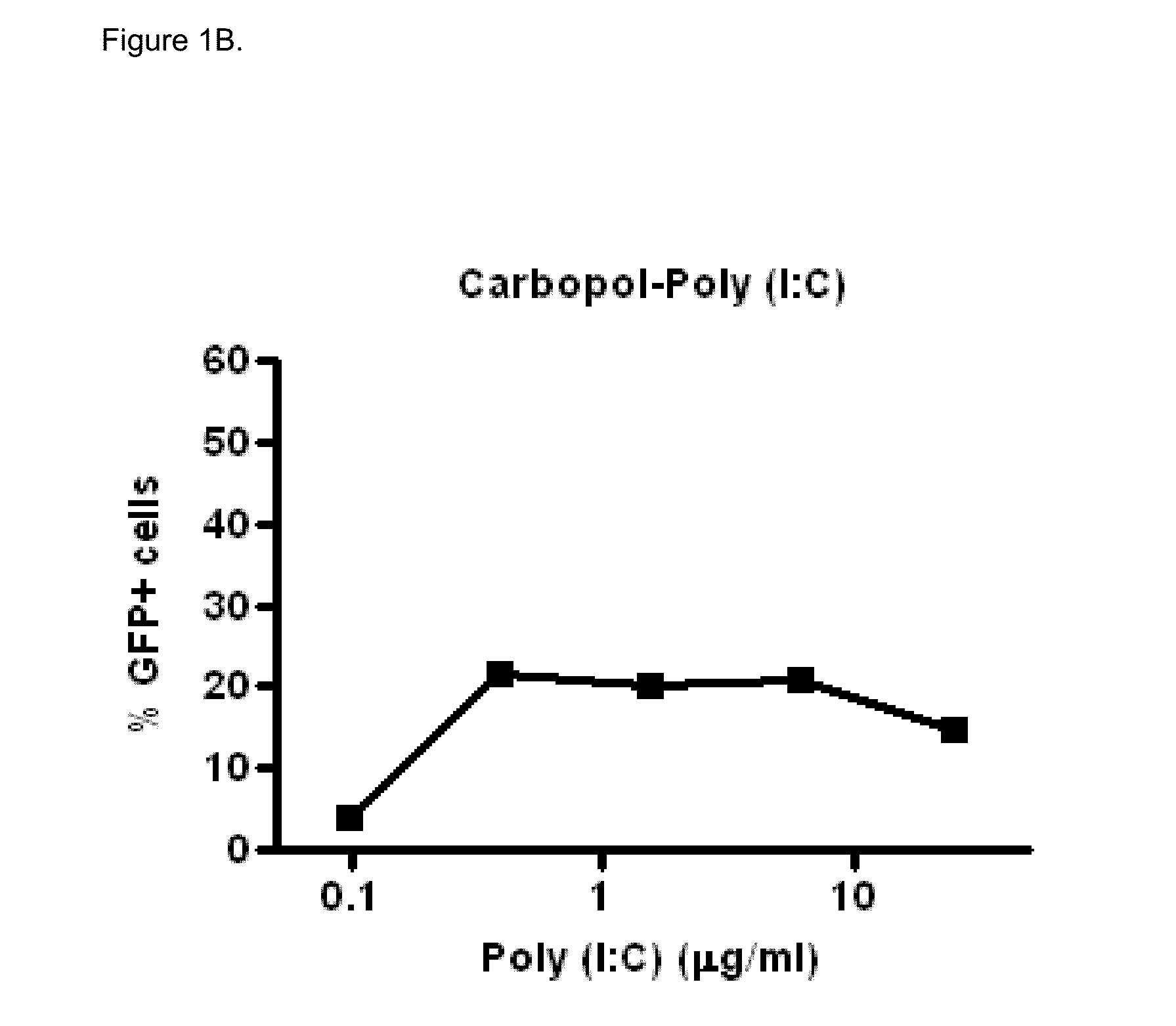
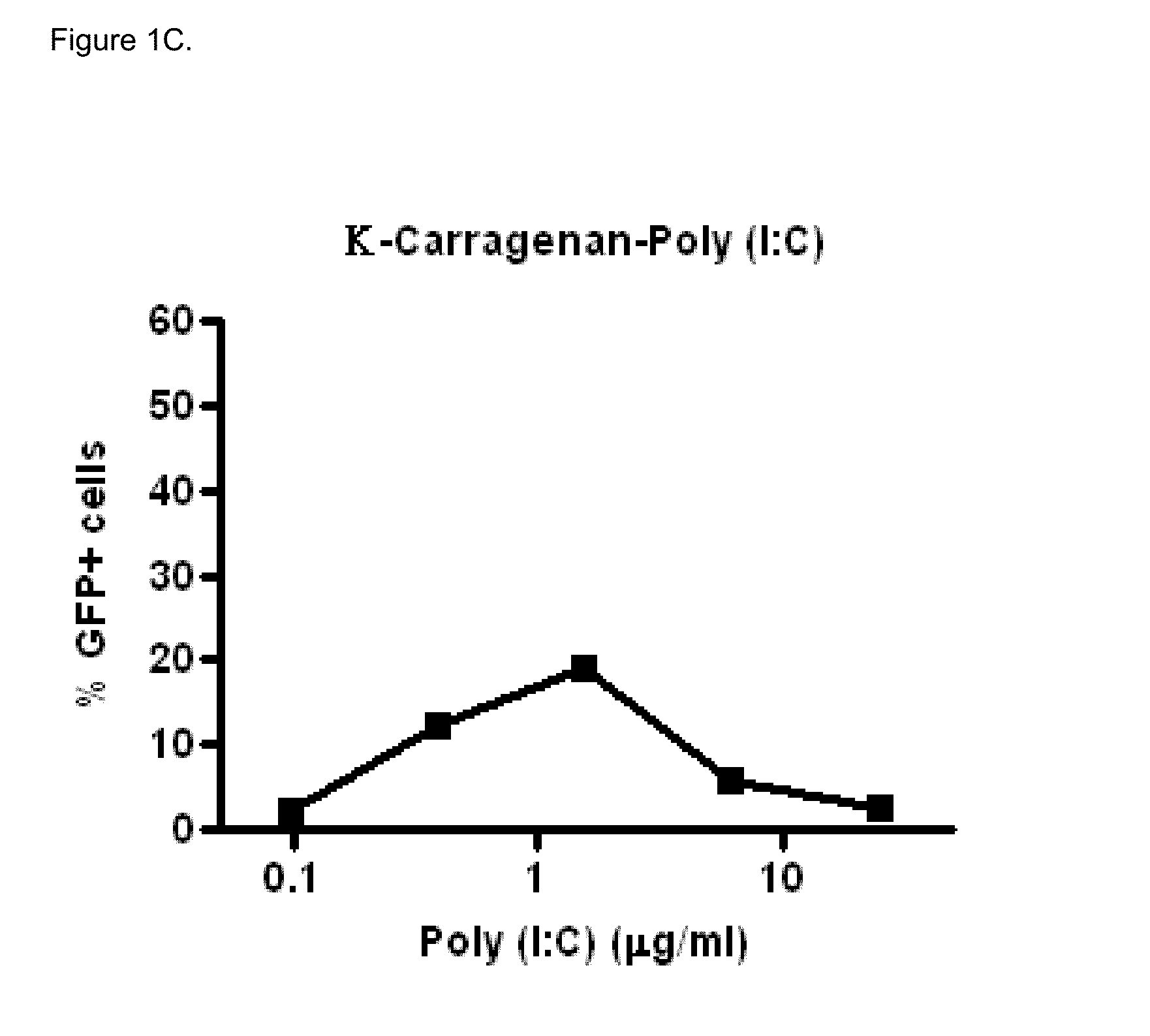
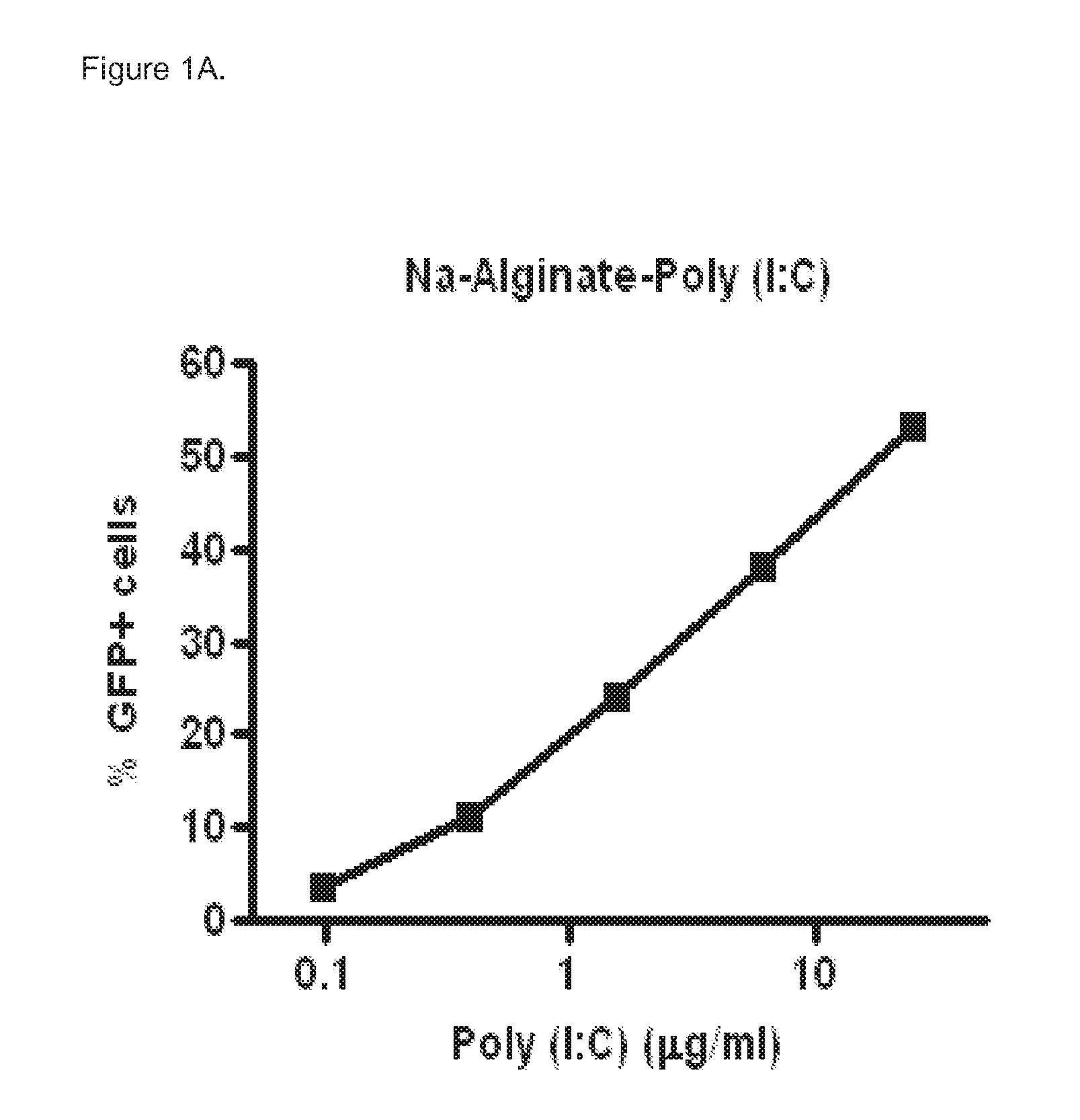
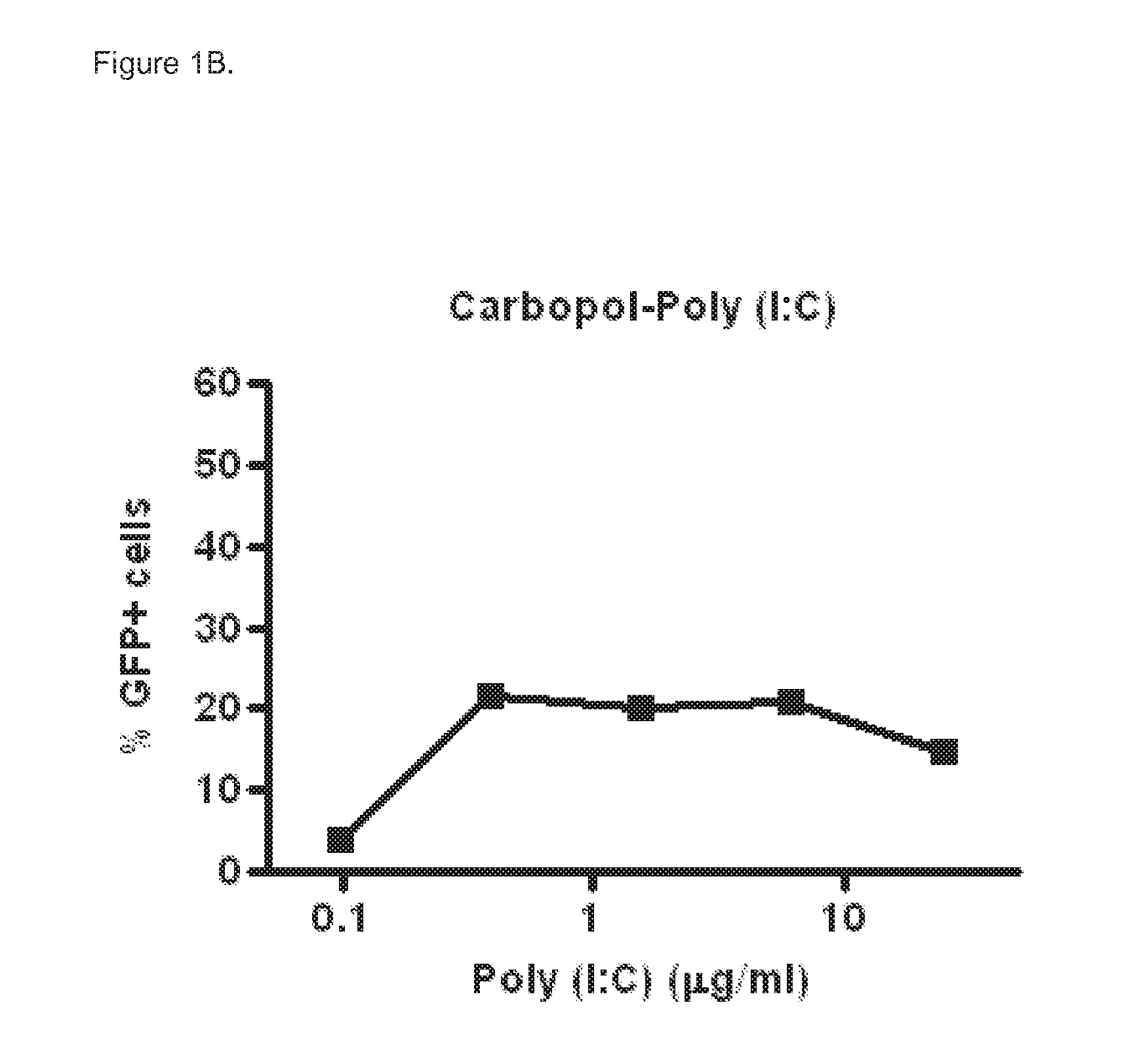
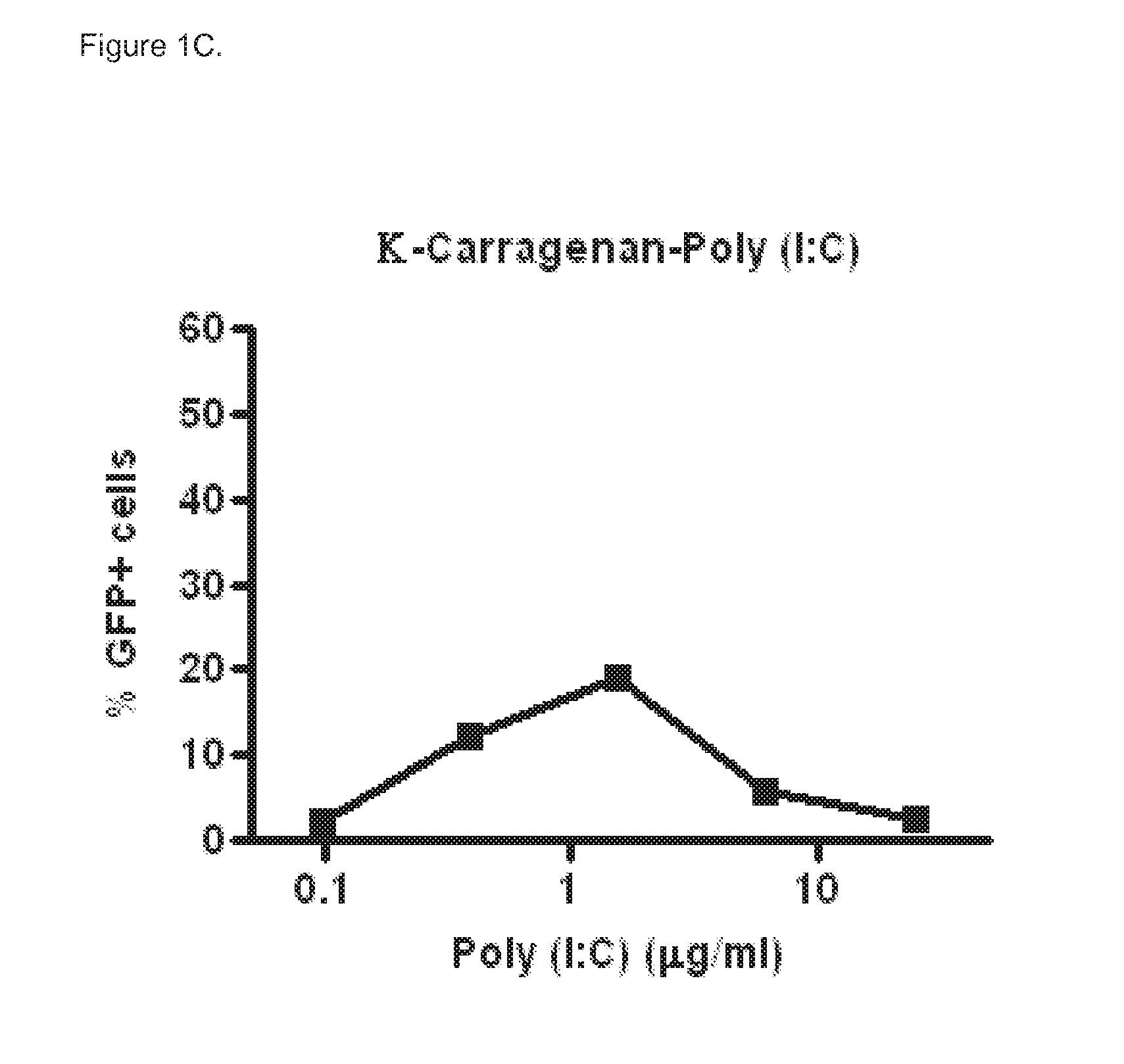
Polyinosinic-Polycytidylic Acid (Poly (I:C)) Formulations for the Treatment of Upper Respiratory Tract Infections
The present invention concerns a composition comprising micro particles of polyinosinic-polycytidylic acid (Poly (I:C)) and a carrier polymer selected from starch, alginate, blanose or DPPC (dipalmitoylphosphatidylcholine) for use in preventing and / or treating viral infections of the upper respiratory tract or the common cold and a device, preferably a nasal delivery system, comprising said composition for use by a patient in need to prevent and / or treat infections or the common cold.
Owner:JANSSEN SCI IRELAND UC
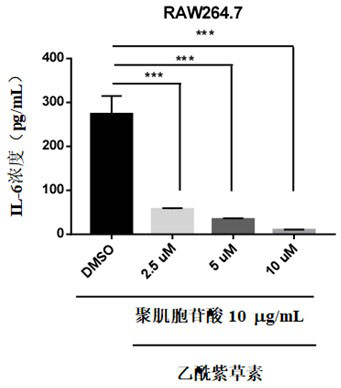
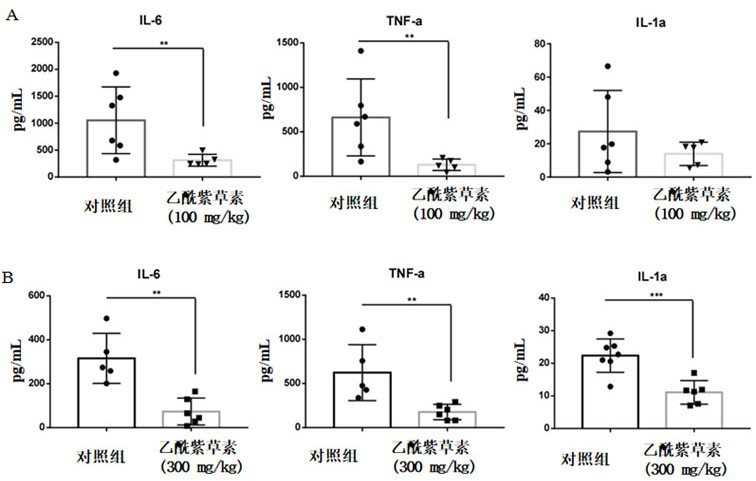
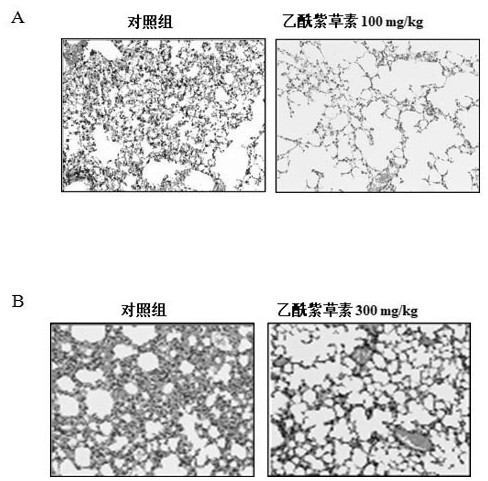
Application of acetylshikonin in preparation of medicine for resisting lung inflammatory factor storm
PendingCN114159420AImprove acute respiratory distress syndromeAntibacterial agentsOrganic active ingredientsInflammatory factorsWhite blood cell
The invention relates to an application of a compound acetylshikonin in preparation of a medicine for resisting lung inflammatory factor storm, namely an inhibition effect of acetylshikonin on mouse acute lung injury and inflammatory factor storm induced by polyinosinic-cytidylic acid and SARS-CoV-2 spinous process protein. The compound acetylshikonin can inhibit inflammatory factors induced by polyinosinic-cytidylic acid and SARS-CoV-2 spinous process protein, obviously inhibits expression of interleukin IL-6 in inflammatory reaction, and can be further applied to research and development of drugs for treating or preventing lung inflammation. The acetylshikonin is suitable for treating lung inflammatory factor storm and acute lung injury caused by viral pneumonia, bacterial pneumonia, mycoplasma pneumonia and the like.
Owner:ZHENGZHOU UNIV +1
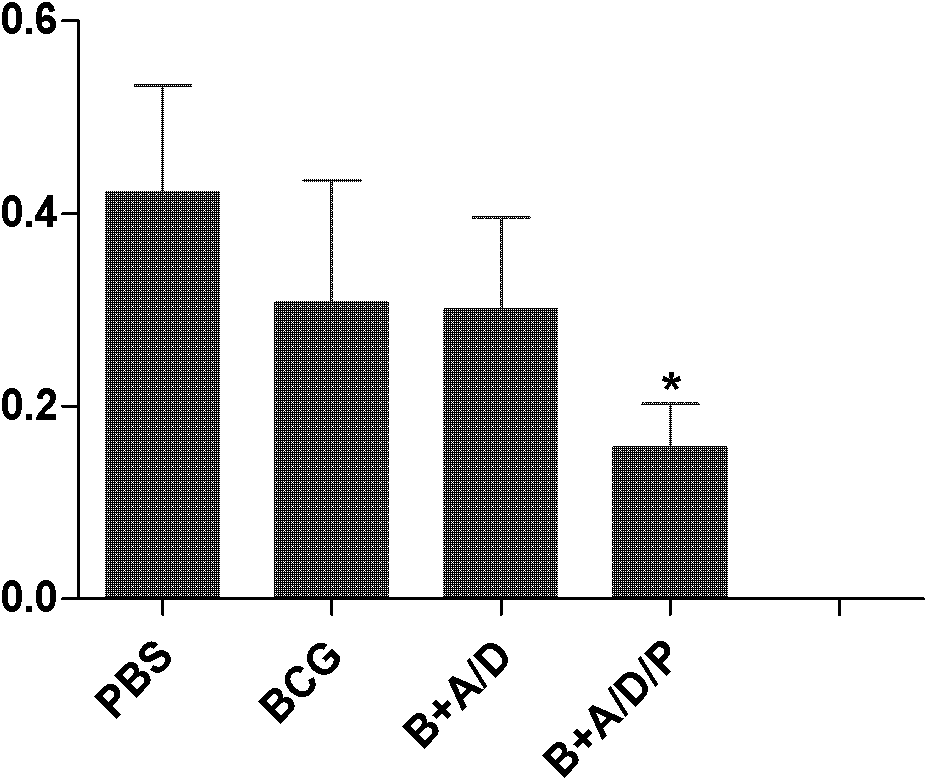
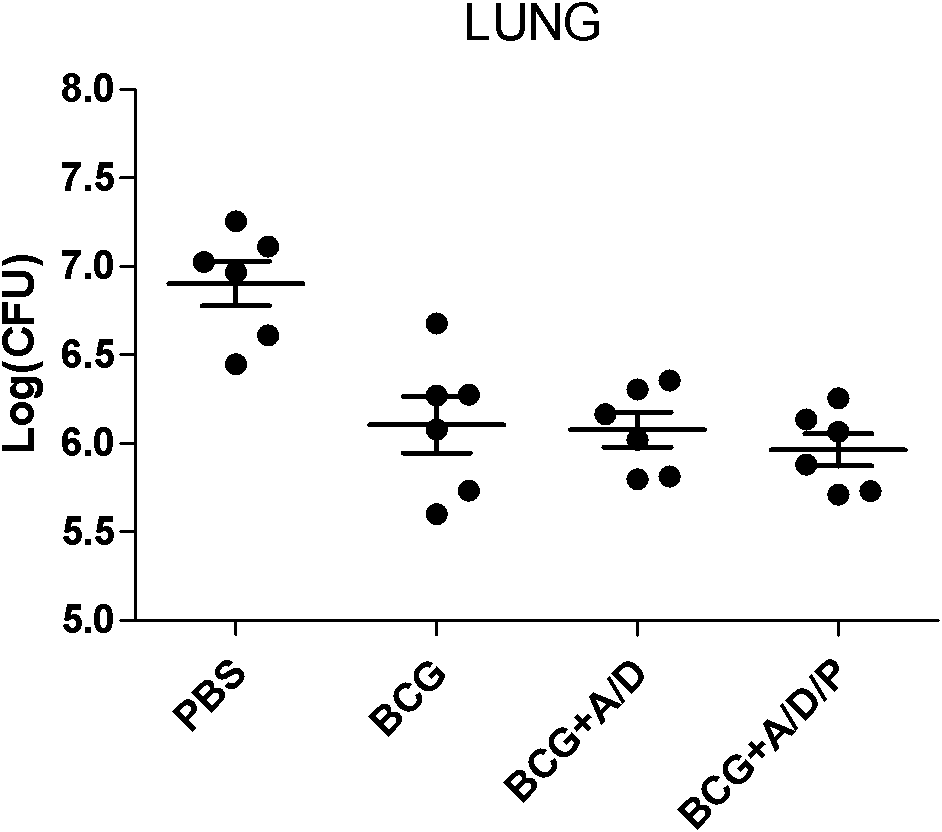
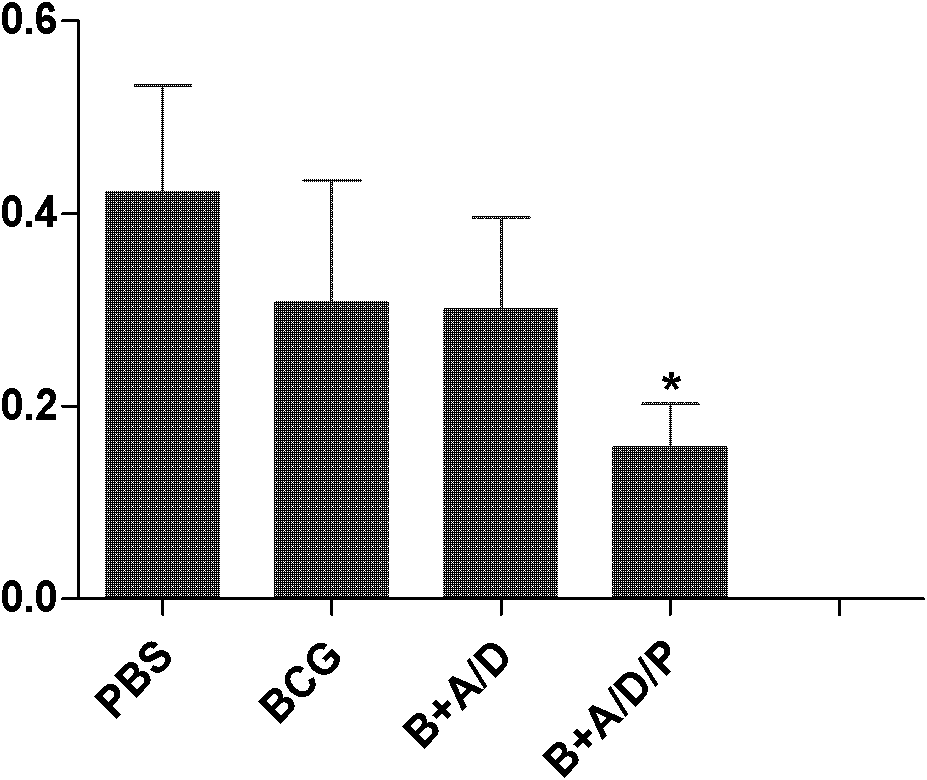
Polyinosinic-polycytidylic acid having long-term unfavorable influence on anti-bacterial defense mechanism
The invention relates to the field of biological medicine, and especially relates to polyinosinic-polycytidylic acid having long-term unfavorable influence on an anti-bacterial defense mechanism. According to the invention, after bacteria infection, influence of polyinosinic-polycytidylic acid administration on bacteria clearance and animal lung bacteria amount of polyinosinic-polycytidylic acid administration are obviously increased, polyinosinic-polycytidylic acid enhances the susceptibility of bacteria on lung tissue, a proportional relation of damage degree on bacteria removing force and continuous time of the proportional relation administration is established, and the polyinosinic-polycytidylic acid has influence on anaphase bacteria removing force. The long-term unfavorable influence of polyinosinic-polycytidylic acid on the lung antibiosis defense mechanism is confirmed.
Owner:宁波美丽人生医药生物科技发展有限公司
A kind of preparation method of double chain polymyocyte dry powder
ActiveCN103599071BQuality improvementImprove stabilityPowder deliveryCosmetic preparationsSide effectPhosphate
The invention provides a preparation method of polyinosinic-polycytidylic acid dry powder. The method comprises the following steps: (1) respectively dissolving polyinosinic acid and polycytidysic acid in a phosphate buffer solution, mixing the solution, adding a stabilizer, uniformly mixing the stabilizer and the solution, and performing heat preservation on the mixture at the temperature of 40-100DEG C; (2) naturally cooling the reaction liquid obtained in the step (1), mixing the reaction liquid with an organic solvent, standing for precipitating, and drying the precipitate, thereby obtaining the polyinosinic-polycytidylic acid dry powder. The preparation method has the beneficial effects that the prepared polyinosinic-polycytidylic acid dry powder has a complete double-helix structural features and physiological activity, the quality and the stability are better than those of the polyinosinic-polycytidylic acid at a solution state, a stronger enzymolysis-resisting performance can be achieved compared with the bare polyinosinic-polycytidylic acid after entering a living body, so that the curative effect can be enhanced. The polyinosinic-polycytidylic acid is purified in the process for making the polyinosinic-polycytidylic acid solution into the dry powder, a plurality of small-molecular-weight substances and impurities can be removed, the toxic and side effects can be reduced, and the quality of the polyinosinic-polycytidylic acid can be improved.
Owner:美亚药业海安有限公司
An adjuvanted Riemeria anatipestifer shadow vaccine with chitosan oligosaccharide
InactiveCN104971346BAvoid the stress of immunizationImprove the level ofAntibacterial agentsBacterial antigen ingredientsPenicillinLocal immunity
The purpose of the present invention is to provide a riemerella anatipestifer bacterial ghost vaccine loaded with polyinosinic acid-polycytidylic acid having interferon inducing activity and added with chitosan oligosaccharide as an adjuvant. According to the technical scheme, a temperature control expression vector is constructed and is electrotransformed into riemerella anatipestifer protoplast, positive clones are screened and then are subjected to bacterial amplification culture at a temperature of 28 DEG C, lysis gene expression is induced at a temperature of 42 DEG C, polylysine is added to continuously act when the OD600 is no longer be reduced so as to completely lyze the live bacteria, centrifugation is performed to separate the bacteria, the dried bacteria and a 6 mg / mL Poly I:C solution according to an equal ratio so as to make the Poly I:C be embedded into the bacterial ghosts, a 2% chitosan oligosaccharide aqueous solution is added, stirring is performed to form a uniform mixture, sub-packaging is performed into penicillin bottles, and freeze-drying is performed so as to obtain the novel riemerella anatipestifer bacterial ghost vaccine. According to the present invention, the prepared riemerella anatipestifer bacterial ghost vaccine is immunized through drinking water, such that the local immunity on the respiratory tract mucous membrane and the digestive tract mucous membrane can be irritated, the humoral immunity can be stimulated, and the protection effect on the human body is not different from the injection immunity.
Owner:QINGDAO AGRI UNIV
Tumor vaccine in mice and preparation method thereof
PendingCN110917341AInhibit tumor growthOvercome the inability to activate CD4
<sup>+</sup>
Technical flaws in T cellsCancer antigen ingredientsAntineoplastic agentsMHC class IAdjuvant
The invention discloses a tumor vaccine in mice and a preparation method thereof. The vaccine contains saccharomyces cerevisiae shell glucan GP, adjuvant polyinosinic-polycytidylic acid Poly(I:C) andpolypeptide M30, which are bonded in a mass ratio of 1:(20-25):(10-12) respectively. The tumor vaccine in mice and the preparation method thereof can overcome the technical defect that the combinationof an MHC class I molecule and short peptides cannot activate CD4+T cells in the prior art, can achieve the aim of activating the CD4+T cells by introducing the specific tumor adjuvant for the firsttime, so that the tumor vaccine in mice has a function of inhibiting tumor growth; and a new thought and foundation are provided to researches and development of vaccines for treating human tumors.
Owner:天津贝罗尼生物科技有限公司
Application of poly inosinic acid cytidine monophosphate combined with anti-CD47 antibody in tumor treatment
InactiveCN111840541AImprove the activation effectImprove response rateAntibody ingredientsAntineoplastic agentsAntiendomysial antibodiesTumor therapy
The invention discloses an application of polyinosinic acid combined with an anti-CD47 antibody in tumor treatment, which can promote the innate immune function of macrophages so as to improve the activation performance of the anti-CD47 antibody, promote the macrophages to kill tumor cells and improve the response rate of tumor patients to the anti-CD47 antibody. Meanwhile, when the polyinosinic acid is combined with the anti-CD47 antibody to treat tumors, adverse reactions such as anemia and the like caused in the treatment process can be remarkably reduced, and the life quality of a patientand the compliance in the treatment process are improved; more importantly, solid tumors including colon cancer can be treated by combining the Poly I: C immunologic adjuvant with the anti-CD47 antibody for immunotherapy, so that the restriction that the anti-CD47 antibody is difficult to realize an effective treatment effect on the solid tumors in the prior art is broken through; a new research direction is provided for immunotherapy and colon cancer treatment in the prior art, and development of the field of tumor treatment is promoted.
Owner:SUN YAT SEN UNIV
SNP (Single Nucleotide Polymorphism) molecular marker capable of influencing quantity of pig red blood cells
ActiveCN108866211AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationMarker-assisted selectionNucleotide
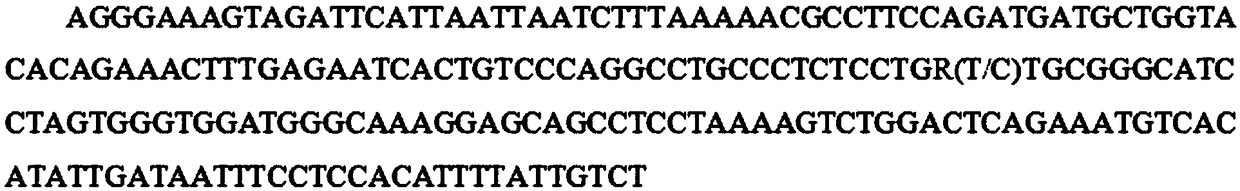
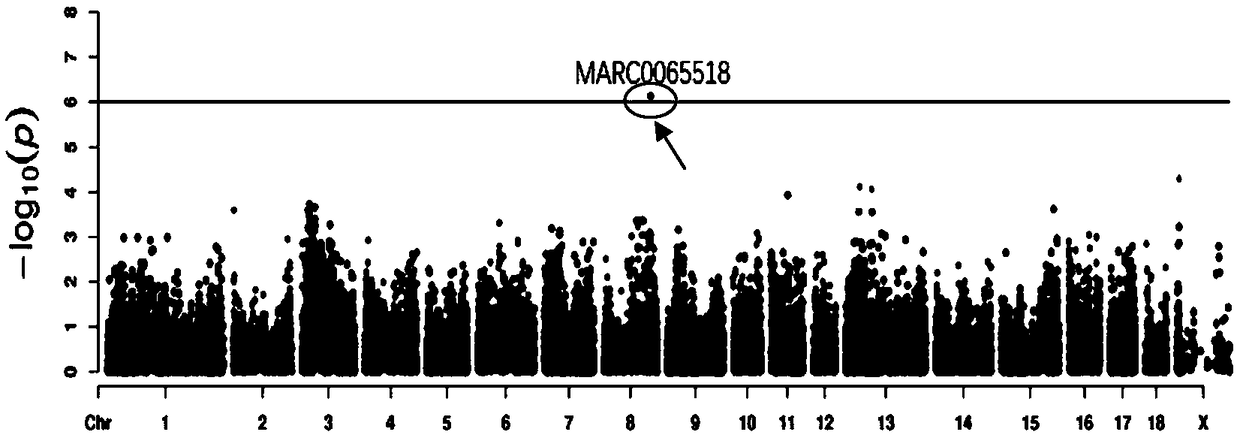
The invention belongs to the field of screening of pig molecular markers and relates to an SNP (Single Nucleotide Polymorphism) molecular marker capable of influencing the quantity of red blood cellsof pigs. The molecular marker is especially correlated with an SNP molecular marker of a red blood cell quantity property of 80-day-old pigs and is cloned from a gene with a registration number of MARC0065518; a gene chip technology is used for carrying out typing screening on the gene to obtain the molecular marker which is correlated with the quantity of the red blood cells of the pigs infectedwith polyinosinic-polycytidylic acid, and a nucleotide sequence is shown as SEQ ID NO: 1; allelic mutation of one T / C occurs on a 101st basic group of the sequence; the SEQ ID NO: 1 sequence has polymorphism due to the mutation. When 101st basic nucleotide on the SEQ ID NO: 1 is C, the pigs have higher red blood cell quantity. The invention discloses a method for screening the molecular marker andapplication of correlation analysis of the molecular marker. The invention provides the novel SNP molecular marker for immunological properties of the pigs, especially marker-assisted selection of the red blood cell quantity.
Owner:HUAZHONG AGRI UNIV
Toll-like receptor agonist mouthwash for preventing and treating oral ulcer
InactiveCN108498362AIncrease heightImprove repair effectCosmetic preparationsToilet preparationsChlorhexidine AcetateOral ulcers
The invention discloses Toll-like receptor agonist mouthwash for preventing and treating oral ulcer. The mouthwash is mainly prepared from raw materials as follows: triacyl lipopeptide, polyinosinic acid-polycytidylic acid, imiquimod, non-methylated oligodeoxynucleotide, chlorhexidine acetate, sodium cyclamate, a moisturizer, mint essential oil, zinc citrate, thymol and water. The Toll-like receptor agonist mouthwash comprises scientifically matched and coordinated raw materials and has inflammation diminishing and sterilization functions, Toll-like receptor agonists in the raw materials regulate local immune functions of organisms, enhance cell proliferation and repair capacity of oral mucosa and promote healing of oral ulcer, and the effect of treating symptoms and root causes is realized.
Owner:睿欧生物科技(上海)有限公司
Immunopotentiator for swine fever live vaccine and preparation method thereof
ActiveCN108635578AAvoid latent infectionLong-lasting immunitySsRNA viruses positive-senseViral antigen ingredientsPig farmsDuration period
The invention discloses an immunopotentiator for a swine fever live vaccine. The immunopotentiator comprises liquid containing corynebacterium parvum (CP) immune particles and solvent,the corynebacterium parvum particle liquid is mainly composed of Tween-80,astragalus polysaccharide,poly I:C (Poly I:C),ethylene diamine tetraacetic acid,manganese gluconate,potassium iodide and corynebacterium parvum preparation (CPP) according to suitable proportions and medicine compatibility. Compared with the prior art,the prepared immunopotentiator for the swine fever live vaccine can effectively inhibit construction and re-activation of swine fever virus latent infection,generate short-term immunity through induction,improve the immune effect of the swine fever vaccine,prolong the immune duration period,effectively avoid the latent infection of the swine fever virus,provide guarantee for prevention and eradication of the swine fever,be widely applied to prevention and treatment of swine fever in pig farms,and improve the economic benefits of the pig farms.
Owner:SHANGHAI CHUANG HONG BIOTECH
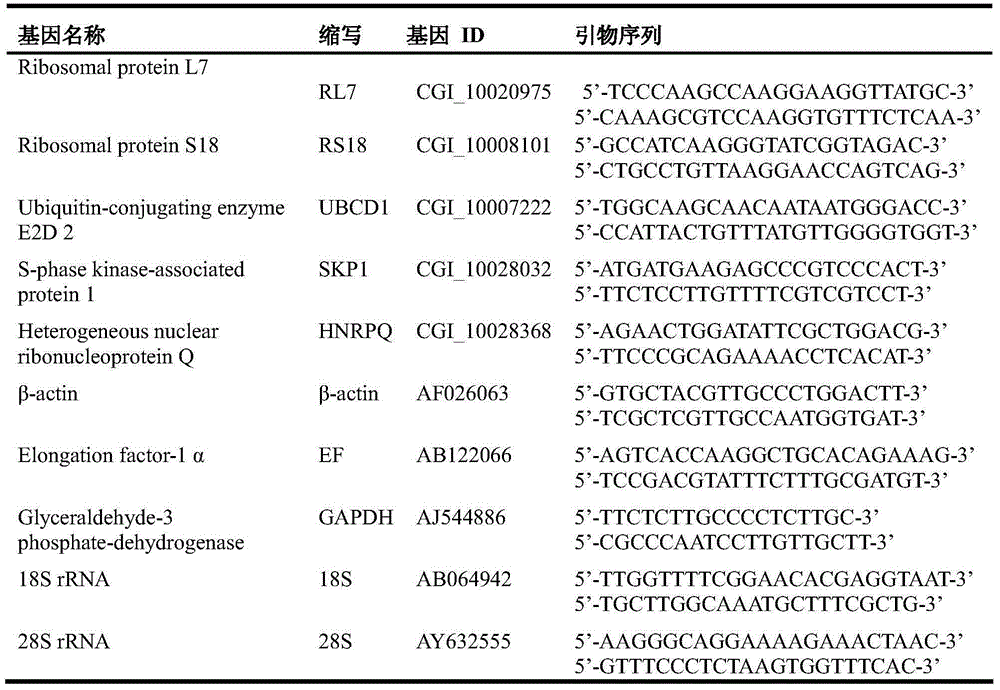
Internal Reference Genes, Primers and Applications of Fluorescence Quantitative Quantification of Oyster oyster poly(i:c) Stress Experiment
ActiveCN104073557BReliable dataImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationReference genesFluorescence
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Preparation method of poultry specific transfer factors
Owner:TIANJIN ASIAN BIOENG
Polyinosinic-polycytidylic acid (poly (i:c)) formulations for the treatment of upper respiratory tract infections
The present invention concerns a composition comprising micro particles of polyinosinic-polycytidylic acid (Poly (I:C)) and a carrier polymer selected from starch, alginate, blanose or DPPC (dipalmitoylphosphatidylcholine) for use in preventing and / or treating viral infections of the upper respiratory tract or the common cold and a device, preferably a nasal delivery system, comprising said composition for use by a patient in need to prevent and / or treat infections or the common cold.
Owner:JANSSEN SCI IRELAND UC
Application of polyinosinic acid in preparation of biological agent for improving response level of HBsAg positive mother infant hepatitis B vaccines
PendingCN113398261ARaise the ratioEnhance immune responseViral antigen ingredientsDigestive systemDendritic cellTrophoblast
The invention provides application of polyinosinic acid in preparation of a biological agent for improving the response level of HBsAg positive mother infant hepatitis B vaccines, and belongs to the technical field of biological agents. The invention discloses application of polyinosinic acid in preparation of a biological agent for improving the response level of HBsAg positive mother infant hepatitis B vaccines. HBeAg of an HBsAg positive mother inhibits a TLR3 signal channel, and placenta immunity is influenced to reduce the immune level of an infant, so that no / weak response of a hepatitis B vaccine is caused. The polyinosinic acid activates a trophoblast cell TLR3 signal channel to increase secretion of IL-2, increase the proportion of CD4 + T cells in PBMC, reduce the proportion of Treg cells and improve the immune level of the body, and rHBsAg stimulation can improve the expression quantity of IL-6 and the proportion of myelodendritic cells (mDC), reduce the expression of IL-10 and improve the anti-HBs level.Therefore, therefore the polyinosinic acid is beneficial to improving the response level of the body to the hepatitis B vaccines.
Owner:SHANXI MEDICAL UNIV
Drug and method for proliferating natural killer cells
InactiveUS20080287365A1Easy activityEasy to collectOrganic active ingredientsPeptide/protein ingredientsNatural Killer Cell Inhibitory ReceptorsToll-like receptor
Natural Killer (NK) cells are obtained by administering a Toll-like receptor ligand such as polyinosinic-polycytidylic acid into the peritoneal cavity of an animal to which lactoferrin has been administered to proliferate NK cells in the peritoneal cavity and collecting NK cells from the peritoneal cavity.
Owner:MORINAGA MILK IND CO LTD
Drug and method for proliferating natural killer cells
InactiveUS20070116675A1Enhances NK activityNK activity be easilyBiocideOrganic active ingredientsNatural Killer Cell Inhibitory ReceptorsToll-like receptor
NK cells are obtained by administering a Toll-like receptor ligand such as polyinosinic-polycytidylic acid into the peritoneal cavity of an animal to which lactoferrin has been administered to proliferate NK cells in the peritoneal cavity and collecting NK cells from the peritoneal cavity.
Owner:MORINAGA MILK IND CO LTD
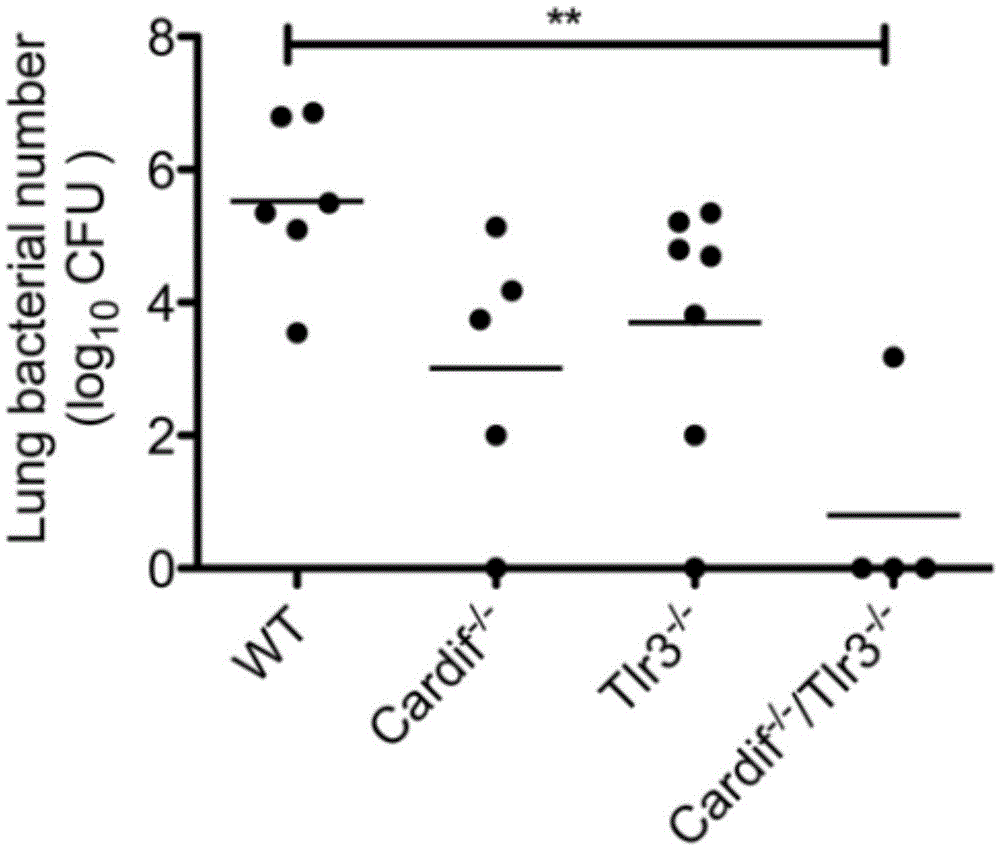
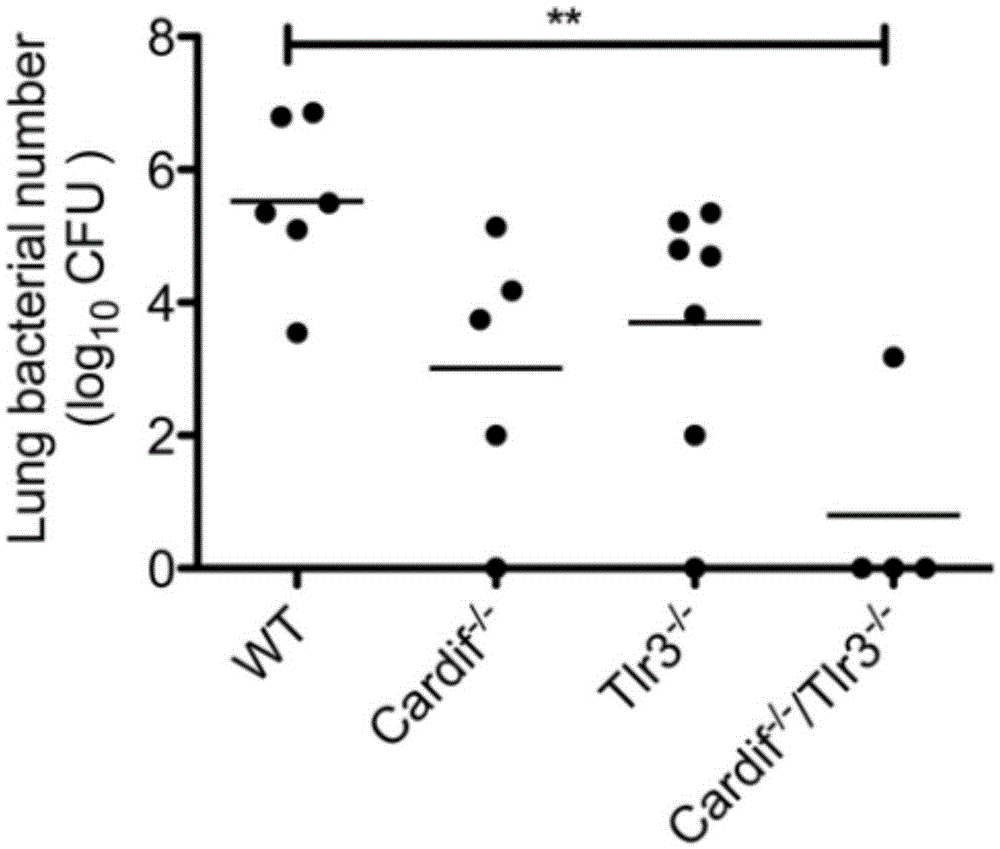
Beneficial influence of activation of TLR3 and RIG-I signaling pathways on bacterium-elimination barrier effect caused by polyinosinic-polycytidylic acid
The invention especially relates to beneficial influence of activation of TLR3 and RIG-I signaling pathways on bacterium-elimination barrier effect caused by polyinosinic-polycytidylic acid, belonging to the field of biological medicine. According to the invention, a virus-immune mouse model is simulated, and TLR3-gene-knocked mice, Cardif-gene-knocked mice and TLR3-and-Cardif-gene-knocked mice are dosed with polyinosinic-polycytidylic acid; and final results prove that activation of the TLR3 and RIG-I signaling pathways exerts effect in alleviating bacterium-elimination barrier effect on the lung of a host caused by polyinosinic-polycytidylic acid.
Owner:宁波美丽人生医药生物科技发展有限公司
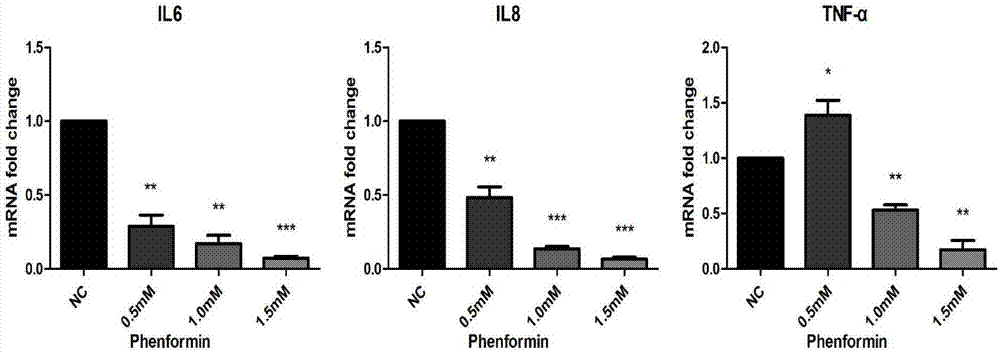
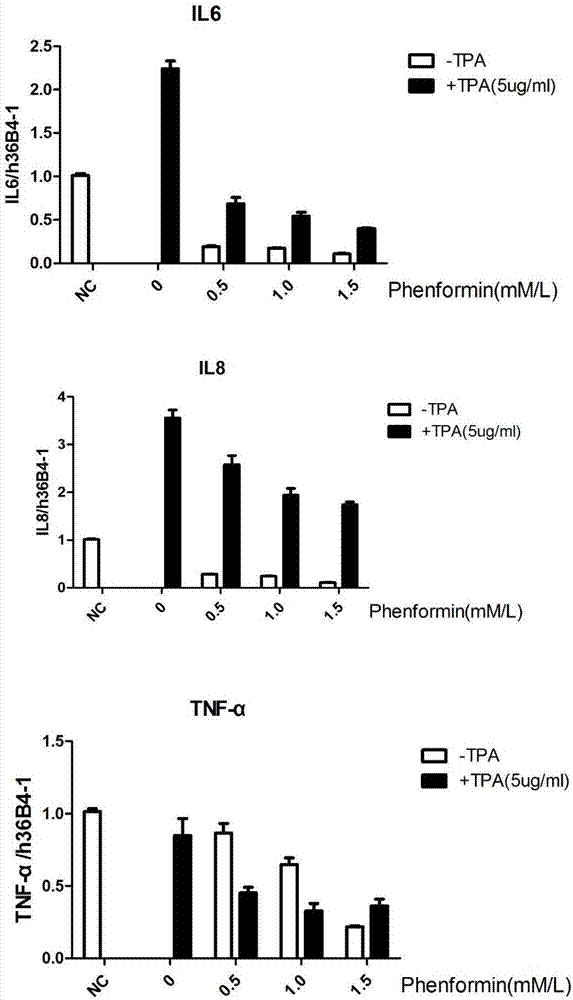
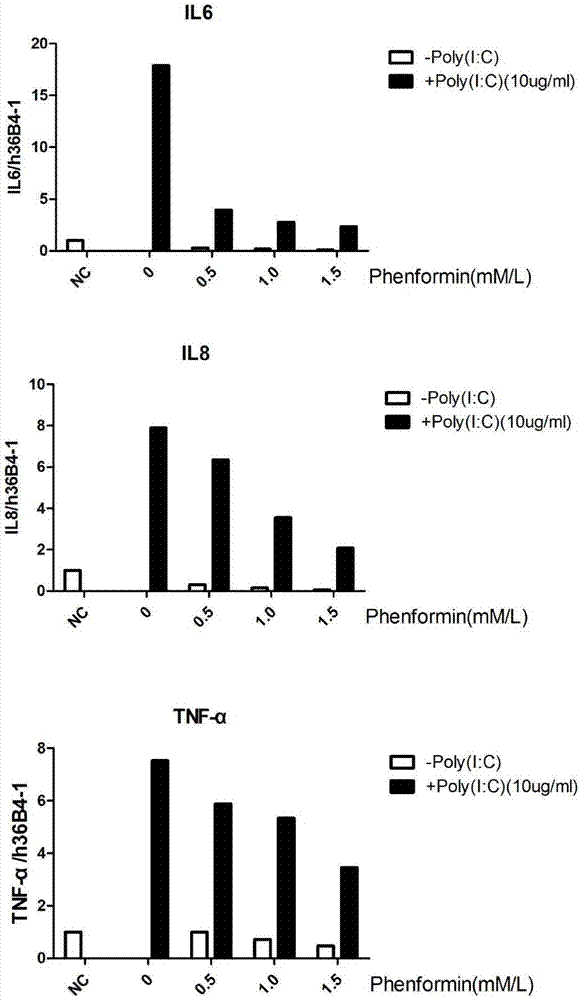
Application of phenformin as skin anti-inflammatory preparation
The invention discloses application of phenformin as a skin anti-inflammatory preparation. First discovery finds that the phenformin can be used for obviously inhibiting skin epidermic cells from secreting various inflammatory cell factors including 6 / 8(IL-6, IL-8) and tumor necrosis factors (TNF alpha), and similarly inflammatory response of human skin epithelial cells induced by frequently-used 12-oxy-tetradecanoyl phorbol-13-acetate (TPA) and poly-poly(C) polyinosinic-polycytidylic acid (poly I:C) (immunologic stimulant), so that the phenformin can be used for preventing and curing related diseases of skin inflammation. Therefore, novel application is developed for the phenformin, and new prevention and treatment means is provided for related diseases of skin inflammation.
Owner:SHANDONG UNIV
Medicament of dsRNA and saikosaponin
InactiveCN103751237ASuppress reproductionAvoid side effects of transient hypothermiaOrganic active ingredientsAntiviralsAdditive ingredientMedicine
The invention relates to a medicament of dsRNA and saikosaponin and application thereof, in particular to a medicament applied to prevention and treatment of viral infection of cultured animals, a preparation method and stability control method of the medicament. The medicament comprises the following ingredients: 20-50 mug / ml of saikosaponin, bupleurum oil with the content range of ultraviolet absorption value of above 0.45; and 1-3 mg / ml of polyinosinic polycytidylic acid with the molecular weight range of 200-500 bp (agarose gel electrophoresis method). The preparation method of the medicament comprises is in accordance with a water injection production technology: adding proper pharmaceutic adjuvant into the ingredients, preparing liquid, filtering, filling, encapsulating, sterilizing and examining for qualification. The medicament can induce generation of endogenous interferon in animal bodies, thus avoiding virus breeding in the animal bodies.
Owner:胡鲸波
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com