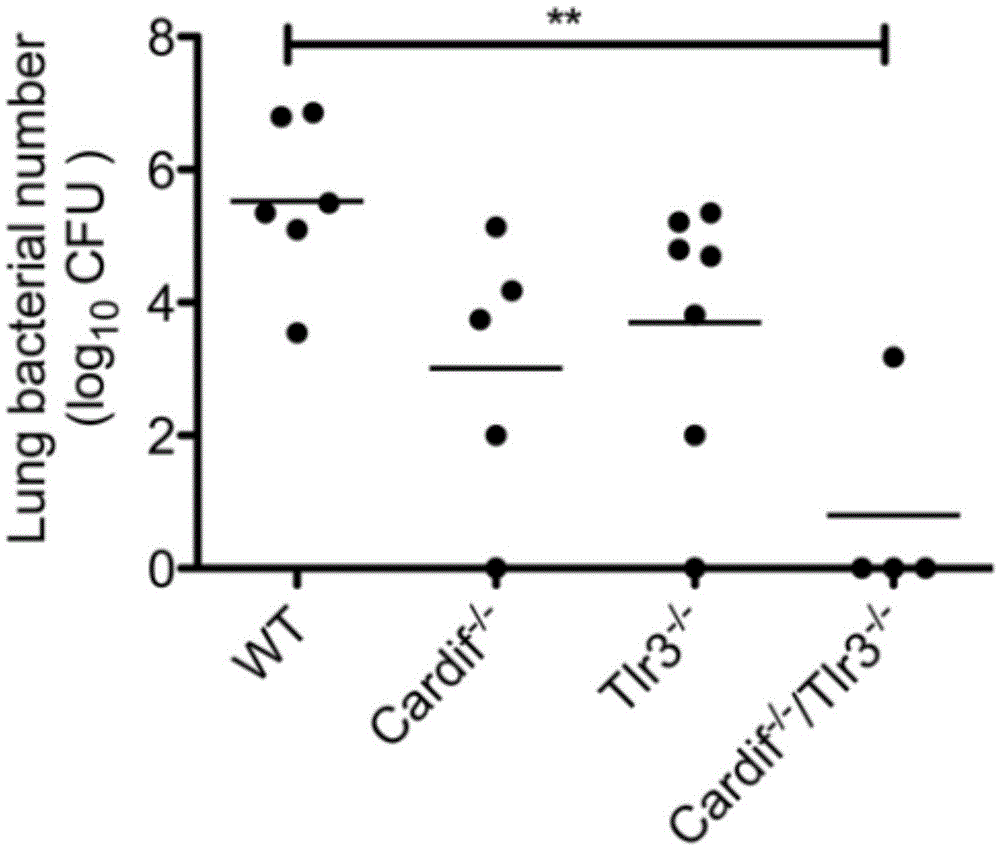
Application of poly inosine-cytidine, imiquimod and gardiquimod in constructing virus immunity mouse model
A technology of polyinosinic acid and immunizing mice, applied in the field of constructing virus immunization mouse models
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
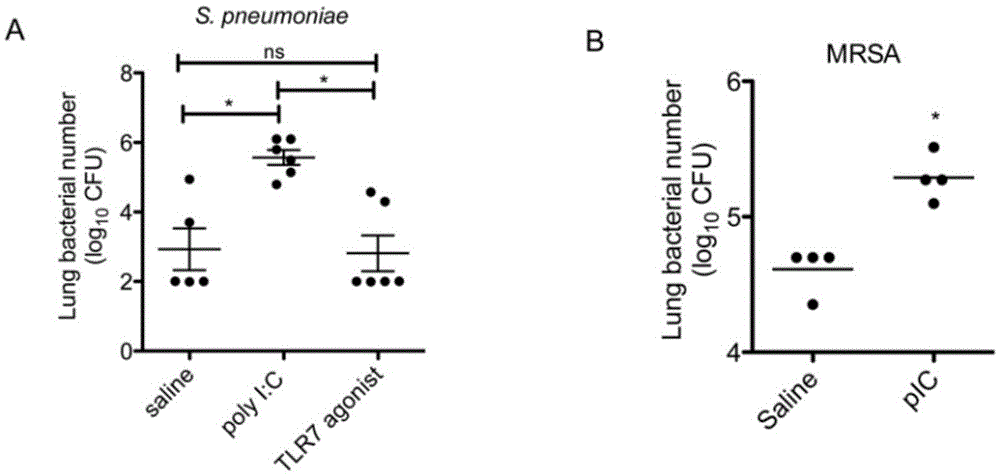
[0052] Polyinosinic acid reduces bacterial clearance
[0053] We first examined the effect of polyinosinic acid administration on bacterial clearance following bacterial infection. The experimental animals were given intranasal administration of polyinosinic acid or imiquimod for two consecutive days (ie, twice). On the third day (i.e., 24 hours after the last polyinosinic acid dose), animals were injected with S. pneumoniae intrathecally. We found a significant increase in bacterial counts in the lungs of animals administered nasal polyinosinic acid. Interestingly, imiquimod or gademotel (TLR7 agonists such as figure 1 A) There was no significant difference in bacterial clearance between the treatment groups. But both groups showed robust clearance during the initial challenge phase. Administration of TLR7 ligand alone was therefore not sufficient to reduce bacterial clearance.
[0054] Second, we examined whether polyinosinic acid also reduced the clearance of another i...
Embodiment 2
[0056] Polyinosinic acid increases susceptibility of lung tissue to bacteria
[0057] Second, we examined whether polyinosinic acid also reduced the clearance of another important clinical pathogen, methicillin-resistant Staphylococcus aureus (MRSA), causing viral secondary bacterial pneumonia. Similar to the above category, after 24h of Staphylococcus aureus infection, polyinosinic acid administration will reduce its clearance rate ( figure 1 B). Thus, polyinosinic acid appears to impair host lung tissue defense mechanisms against two pathogens clinically responsible for viral secondary bacterial pneumonia, Streptococcus pneumoniae and methicillin-resistant Staphylococcus aureus.
Embodiment 3
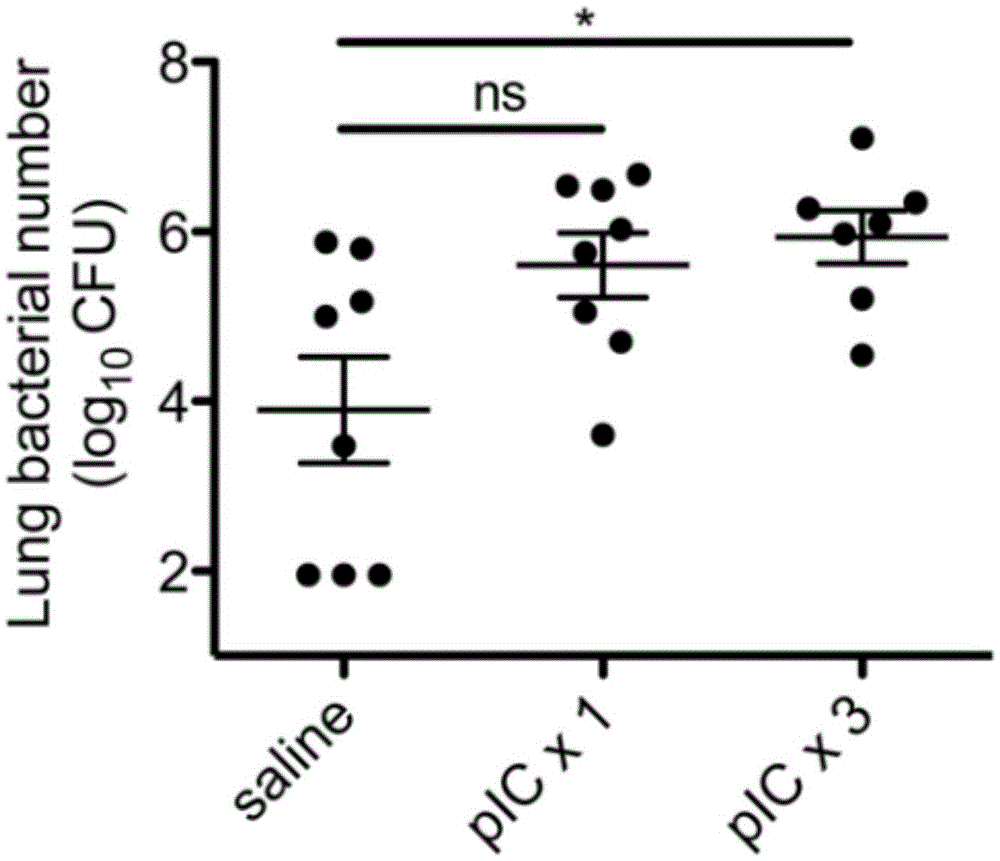
[0059] Duration of Polyinosinic Acid Administration and Risk of Bacterial Infection
[0060] Second, we observed the time point at which the bacterial clearance rate decreased in the animal body after polyinosinic acid administration. Since viral infections, such as influenza, usually last for several days or even longer, we conducted related experimental studies, taking polyinosinic acid in 1 dose or 3 doses to simulate the effect of viral infection. After 24 hours of intranasal administration of polyinosinic acid or saline once or three times, the experimental animals were intrathecally injected with Streptococcus pneumoniae. At 48h, record the amount of bacteria in the body. We found that the dose of polyinosinic acid correlated with the rate of bacterial clearance. The bacterial clearance rate in the experimental animals with one-time administration of polyinosinic acid will tend to decrease (8 times higher than the average CFU value of the normal saline control group, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com