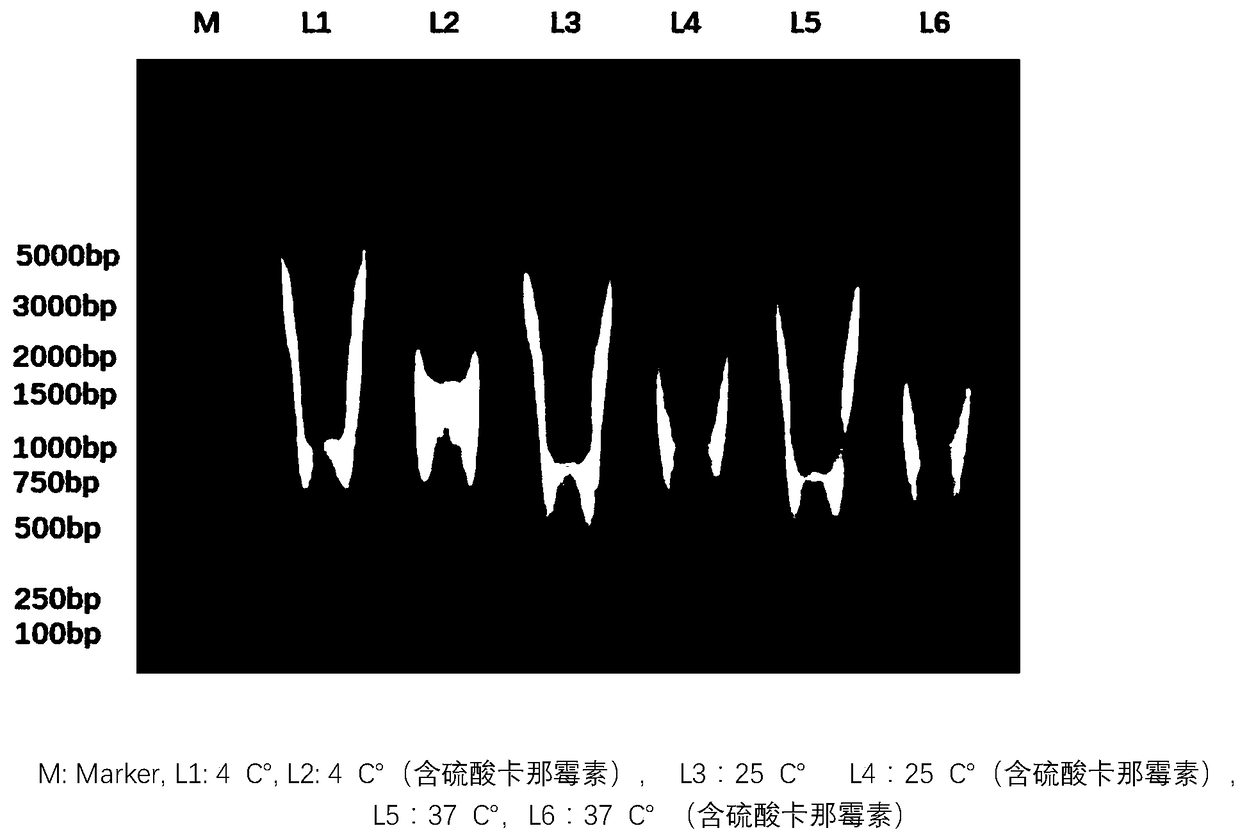
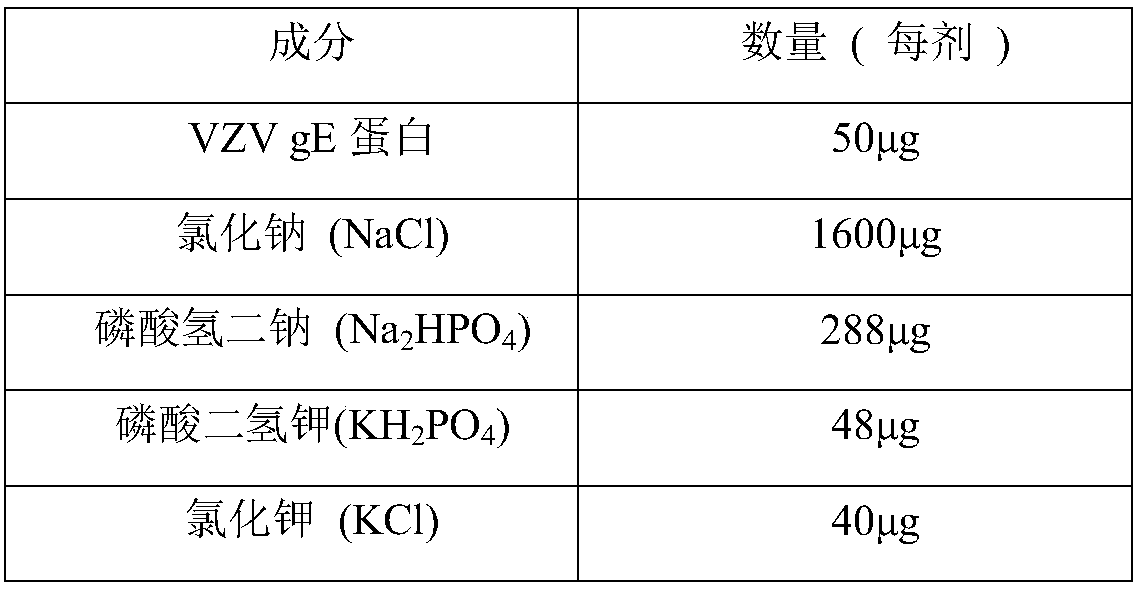
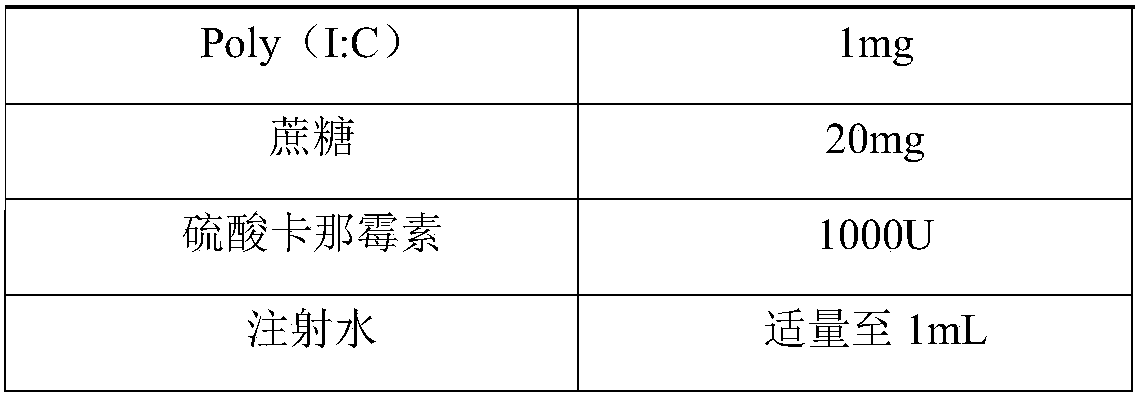
Herpes zoster vaccine, and preparation method and application thereof
A technology of herpes zoster and herpes zoster virus, which is applied in the field of vaccines, can solve problems such as high safety risks of vaccines, and achieve the effects of enhancing cellular and humoral immune responses, improving immune effects, and enhancing Th1 responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1 The preparation of the vaccine containing recombinant varicella zoster virus (VZV) gE protein
[0041] Experimental materials and sources:
[0042] CHO cell line, purchased from Thermo company;
[0043] CD CHO medium was purchased from Thermo Fisher.
[0044] Preparation:
[0045] 1. The recombinant CHO cell line stably expressing the VZV gE protein adopts a well-known method, according to the amino acid sequence of the VZV gE protein found on the NCBI website, synthesizes the gene sequence, and transfects it into CHO cells according to the method described in the "Molecular Cloning Experiment Guide". Monoclonal cells were selected by the limiting dilution method, and after multiple rounds of screening, they were expanded and cultured in cell shake flasks and then frozen. After collecting the supernatant of the subcloned cells, samples were taken for Western Blot detection. According to the gray scale of the bands, the monoclonal cells with higher expres...
Embodiment 2
[0053] Embodiment 2 Preparation of vaccine containing recombinant varicella-zoster virus (VZV) gE protein
[0054] Experimental materials and sources:
[0055] CHO cell line, purchased from Thermo company;
[0056] CD CHO medium was purchased from Thermo Fisher.
[0057] Preparation:
[0058] 1. The recombinant CHO cell line stably expressing the VZV gE protein adopts a well-known method, according to the amino acid sequence of the VZV gE protein found on the NCBI website, synthesizes the gene sequence, and transfects it into CHO cells according to the method described in the "Molecular Cloning Experiment Guide". Monoclonal cells were selected by the limiting dilution method, and after multiple rounds of screening, they were expanded and cultured in cell shake flasks and then frozen. After collecting the supernatant of the subcloned cells, samples were taken for Western Blot detection. According to the gray scale of the bands, the monoclonal cells with higher expression lev...
Embodiment 3
[0066] Embodiment 3 Preparation of vaccine containing recombinant varicella-zoster virus (VZV) gE protein
[0067] Experimental materials and sources:
[0068] CHO cell line, purchased from Thermo company;
[0069] CD CHO medium was purchased from Thermo Fisher.
[0070] Preparation:
[0071] 1. The recombinant CHO cell line stably expressing the VZV gE protein adopts a well-known method, according to the amino acid sequence of the VZV gE protein found on the NCBI website, synthesizes the gene sequence, and transfects it into CHO cells according to the method described in the "Molecular Cloning Experiment Guide". Monoclonal cells were selected by the limiting dilution method, and after multiple rounds of screening, they were expanded and cultured in cell shake flasks and then frozen. After collecting the supernatant of the subcloned cells, samples were taken for Western Blot detection. According to the gray scale of the bands, the monoclonal cells with higher expression lev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com