Tumor vaccine in mice and preparation method thereof
A tumor vaccine and mouse technology, applied in the field of mouse tumor vaccine and its preparation, to achieve the effect of inhibiting tumor growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The embodiment of the present invention discloses a tumor vaccine for mice, wherein the beer yeast shell glucan GP, the adjuvant polyinosinic acid Poly(I:C) and the polypeptide M30 are respectively connected according to the mass ratio of 1:20:10 , to get GP-Poly(I:C)-M30 mouse tumor vaccine.
[0044] Wherein, the preparation method of wine yeast shell glucan GP is as follows:
[0045] (1) Take 5 bags (15g / bag) of Angel high-efficiency active dry yeast and place them in a 1L glass beaker, add 1L deionized water to resuspend the yeast.
[0046] (2) Place the resuspended yeast solution in a centrifuge bottle and balance, centrifuge at 2000rpm for 5min, and discard the supernatant. Repeat the washing twice by adding deionized water again (to remove the additives in the yeast).
[0047] (3) Collect the yeast cells into a beaker, add 1L NaOH (1M / L) and heat and stir at 90°C for 1h on a magnetic stirrer.
[0048] (4) Centrifuge at 5000rpm for 5min to collect the precipit...
Embodiment 2
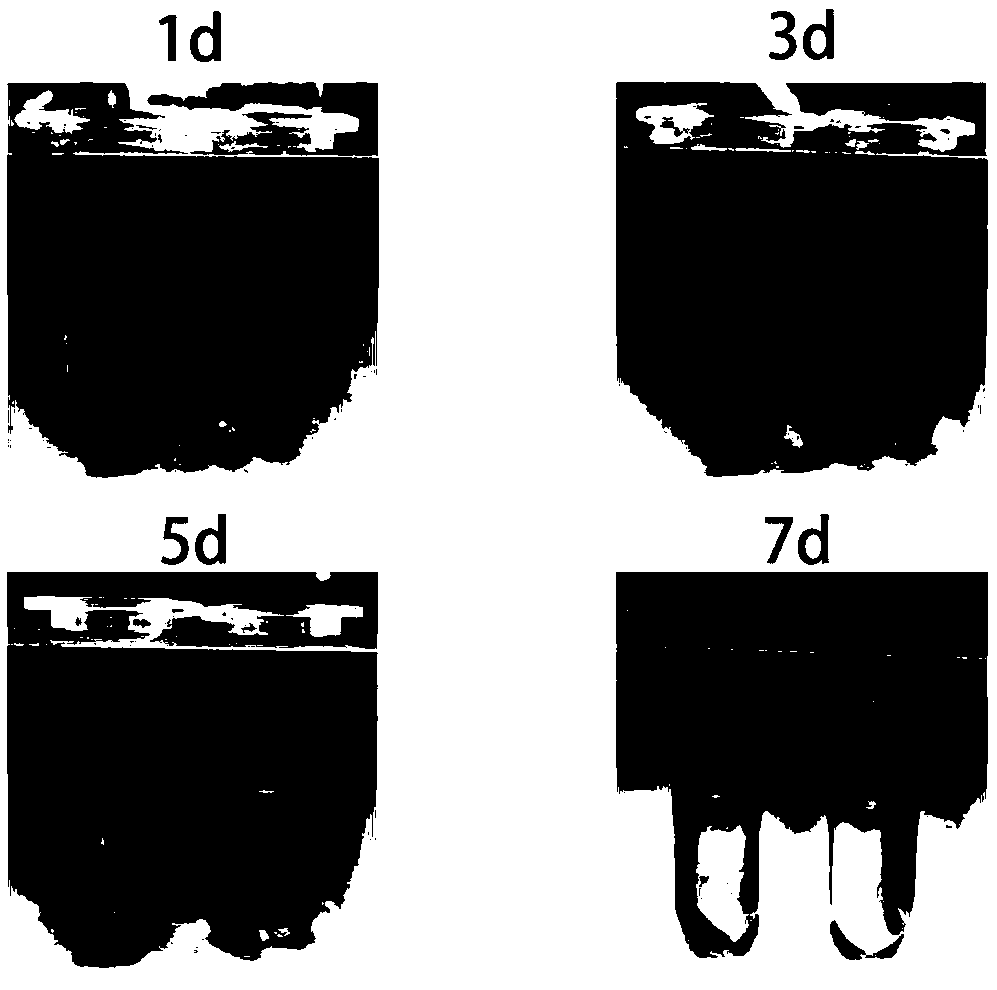
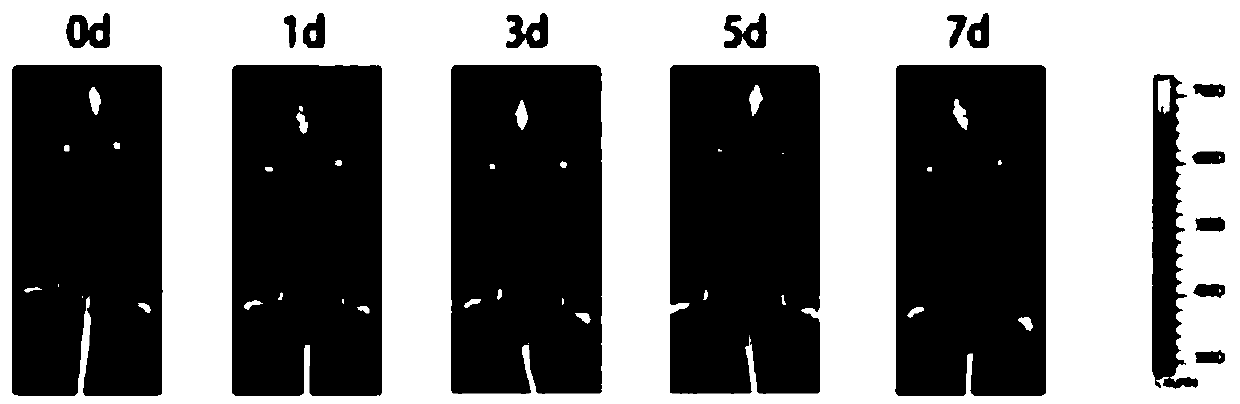
[0061] The embodiment of the present invention discloses a mouse tumor vaccine, wherein the beer yeast shell glucan GP, the adjuvant polyinosinic acid Poly(I:C) and the polypeptide M30 are respectively connected according to the mass ratio of 1:25:11 , to get GP-Poly(I:C)-M30 mouse tumor vaccine. Among them, the structure of beer yeast shell dextran GP under the electron microscope is as follows: figure 1 shown.
[0062] The preparation method is as follows: (1) Weigh 5mg of GP into a 2ml EP tube, add 2ml of oil phase solution (cyclohexane / Igepal = volume ratio 85:15) into the tube and place it in an ultrasonic cleaner for ultrasonic dispersion for 50min.
[0063] (2) After putting magnets into the tube, add 12.5 μl of 10 mg / ml fluorescently labeled polypeptide M30 solution and 5.5 μl of 10 mg / ml Poly(I:C) solution, and stir overnight on a magnetic stirrer.
[0064] (3) Take 10 μl of 0.5% chitosan solution, add it into 250 μl oil phase solution and mix evenly, then mix it ...
Embodiment 3
[0069] The embodiment of the present invention discloses a mouse tumor vaccine, wherein the beer yeast shell glucan GP, the adjuvant polyinosinic acid Poly(I:C) and the polypeptide M30 are connected according to the mass ratio of 1:22:12 respectively , to get GP-Poly(I:C)-M30 mouse tumor vaccine. Among them, the structure of beer yeast shell dextran GP under the electron microscope is as follows: figure 1 shown.
[0070] The preparation method is as follows: (1) Weigh 5 mg of GP into a 2 ml EP tube, add 5 ml of oil phase solution (cyclohexane / Igepal = volume ratio 85:15) into the tube and place it in an ultrasonic cleaner for ultrasonic dispersion for 50 min.
[0071] (2) After putting magnets into the tube, add 11 μl of 10 mg / ml fluorescently labeled polypeptide M30 solution and 6 μl of 10 mg / ml Poly(I:C) solution, and stir overnight on a magnetic stirrer.
[0072] (3) Take 7 μl of 0.5% chitosan solution, add it to 220 μl oil phase solution and mix evenly, then mix it eve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com