Application of polyinosinic-polycytidylic acid (poly I:C) combined dimo-thylidioctyl ammonium bromide (DDA) mixed adjuvant in preparation of tuberculosis subunit vaccines
A dimethylhexadecane and subunit vaccine technology, which is applied to medical preparations containing active ingredients, bacterial antigen components, antibody medical components, etc., can solve the problems of difference in effectiveness and poor protection effect, and achieve relief Pathological damage, enhance secretion level, reduce the effect of lung pathological damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
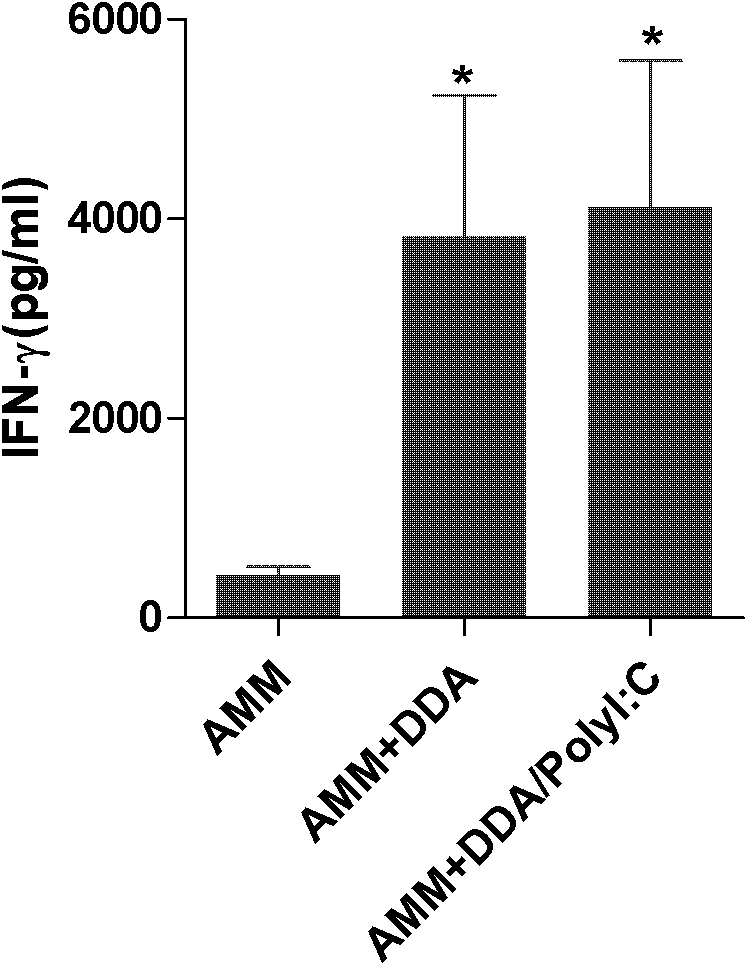
[0026] Dissolve PolyI:C in phosphate buffer to 0.5 mg / ml, dilute the fusion protein AMM to 0.4 mg / ml with sterile PBS, prepare DDA with sterile distilled water to a 2.5 mg / ml solution, bathe in 80°C for 10 minutes, and cool to room temperature; mix 50 μl of PolyI:C solution with an equal amount of AMM protein solution, and place at room temperature for 1 min; add 100??L DDA solution to the mixed solution drop by drop, and then fully emulsify the vaccine to obtain a uniform creamy oil. Subunit vaccine AMM+DDA / PolyI:C.
[0027] The mice in each experimental group were subcutaneously immunized in the groin, and the total volume of the vaccine was 200ul.
[0028] Two vaccine immunization strategies were adopted, one was booster immunization with fusion protein vaccine; the other was booster immunization with fusion protein vaccine after BCG primary immunization.
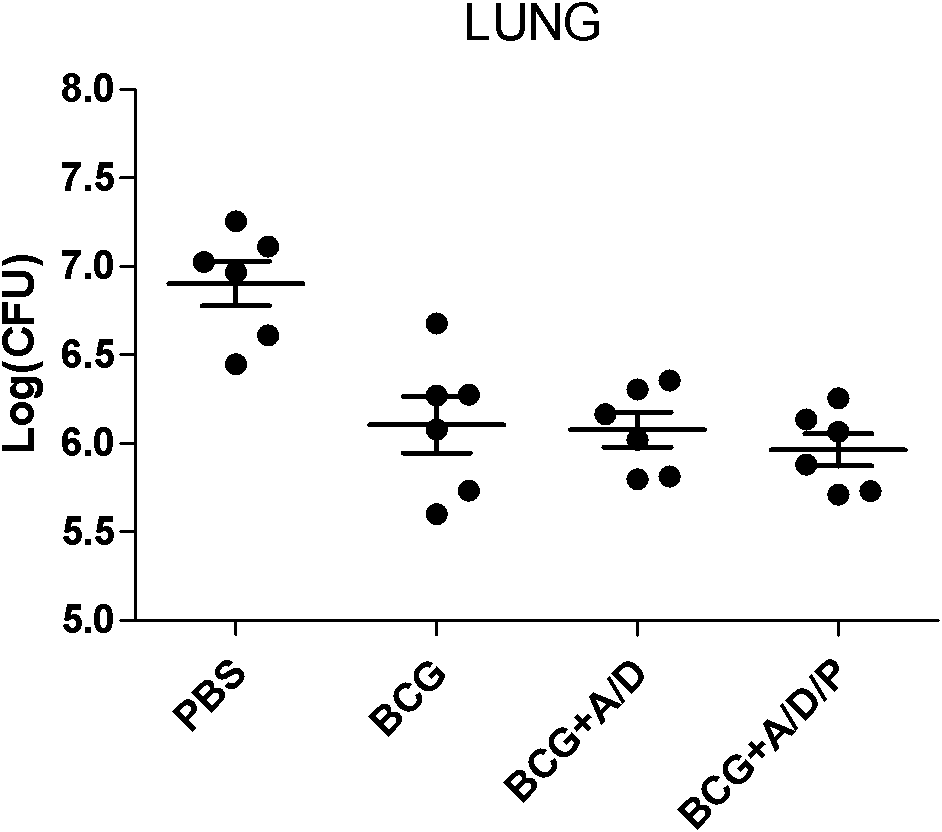
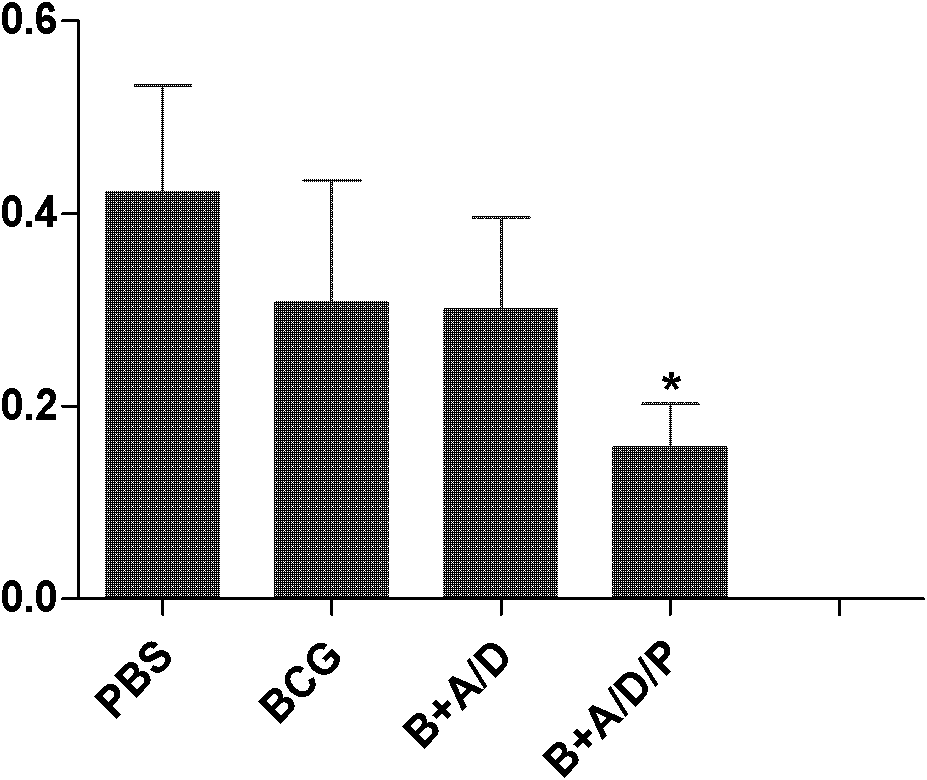
[0029] 1. Fusion protein AMM vaccine group with DDA / PolyI:C as adjuvant (AMM+DDA / PolyI:C), fusion protein AMM vaccine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com