Pharmaceutical composition of dsRNA and astragalus polysaccharide and application thereof
A technology for astragalus polysaccharide and medicine, which is applied in the field of preparation and stability control of the combination medicine, can solve the problems of lack of compatibility of active substances, lack of suitable conditions for double-chain pairing, systematic research on stability influencing factors, etc. The effect of prevention and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0015] Specific embodiment one: this specific embodiment adopts the following technical scheme: it is composed of astragalus polysaccharide and dsRNA according to a certain ratio, and the content range of astragalus polysaccharide: 20-50mg / ml; -5mg / ml, molecular weight range: 200-500bp (agarose gel electrophoresis). The main components of the astragalus polysaccharide and dsRNA are prepared according to the production process of the aqueous injection, and the drug combination is obtained after liquid preparation, filtration, potting, sterilization and passing the inspection. Add a certain amount of polyinosinic acid to water for injection to dissolve at 20°C-60°C, then add equimolar polycytidylic acid to dissolve, keep warm for 20-50 minutes, add an appropriate amount of astragalus polysaccharide, mix well, filter , potting, and sterilization.
[0016] The synthesis and drug combination of polymyocytes is to add 0.3 g of sodium pyrosulfite, 0.6 g of sodium chloride and approp...
specific Embodiment approach 2
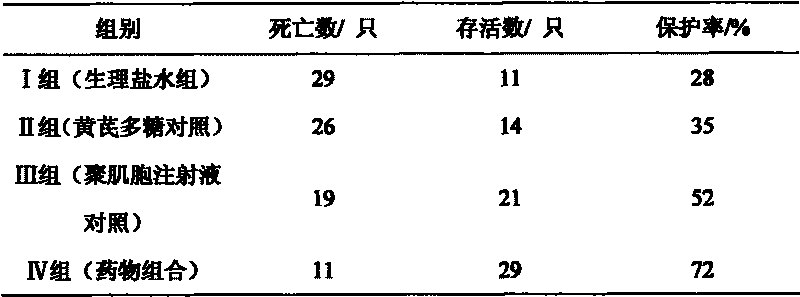
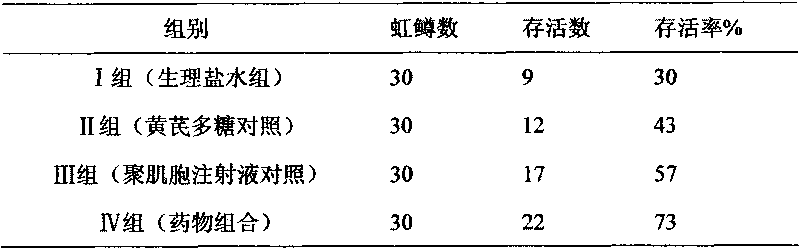
[0020] Embodiment 2: The drug combination is diluted with water for injection to contain 50 μg / ml of polymyocytes. 160 test chicks were randomly divided into 4 test groups, 40 in each group. Group I: blank control group (physiological saline group); Group II: astragalus polysaccharide drug control group (astragalus polysaccharide injection, 20 mg per kilogram of body weight); Every kilogram of body weight 0.05mg) IV group: drug combination test group (per kilogram of body weight 1ml); The above chicks are isolated and raised in cages, and the blank control group is raised in different chicken houses with the positive control group and the drug test group, and the feeding conditions are kept consistent. After feeding for 10 days, each group was immunized with Newcastle disease vaccine. 21 days after immunization, each group will be treated with drugs according to the grouping plan, and will be challenged after 12 hours (100 units of LD 50 24 hours after the challenge, each gr...
specific Embodiment approach 3
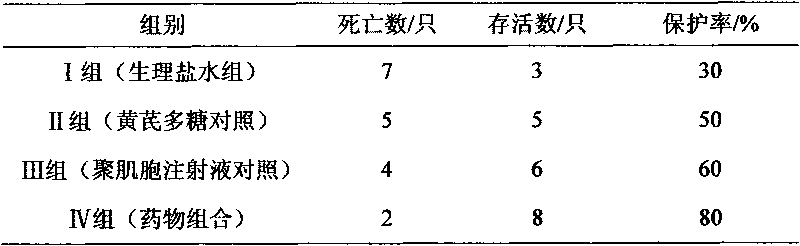
[0023] Embodiment 3: The drug combination is diluted with water for injection to contain 50 μg / ml of polymyocytes. The 40 test piglets were randomly divided into 4 test groups, 10 piglets in each group. The grouping and test methods are as follows: Group I: blank control group (normal saline group); Group II: astragalus polysaccharide drug control group (astragalus polysaccharide injection, 20 mg per kilogram of body weight); Muscle cell injection, 0.05mg per kilogram of body weight) Group IV: drug combination test group (1ml per kilogram of body weight); the weaned piglets were grouped and raised in isolation according to the above experiments, and the feeding conditions were kept consistent. After feeding for 1 week, each group was immunized with classical swine fever vaccine (the immunization dose was the conventional immunization dose). After 21 days of immunization, each group was treated with drugs according to the grouping plan, and challenged after 12 hours (the dose ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com