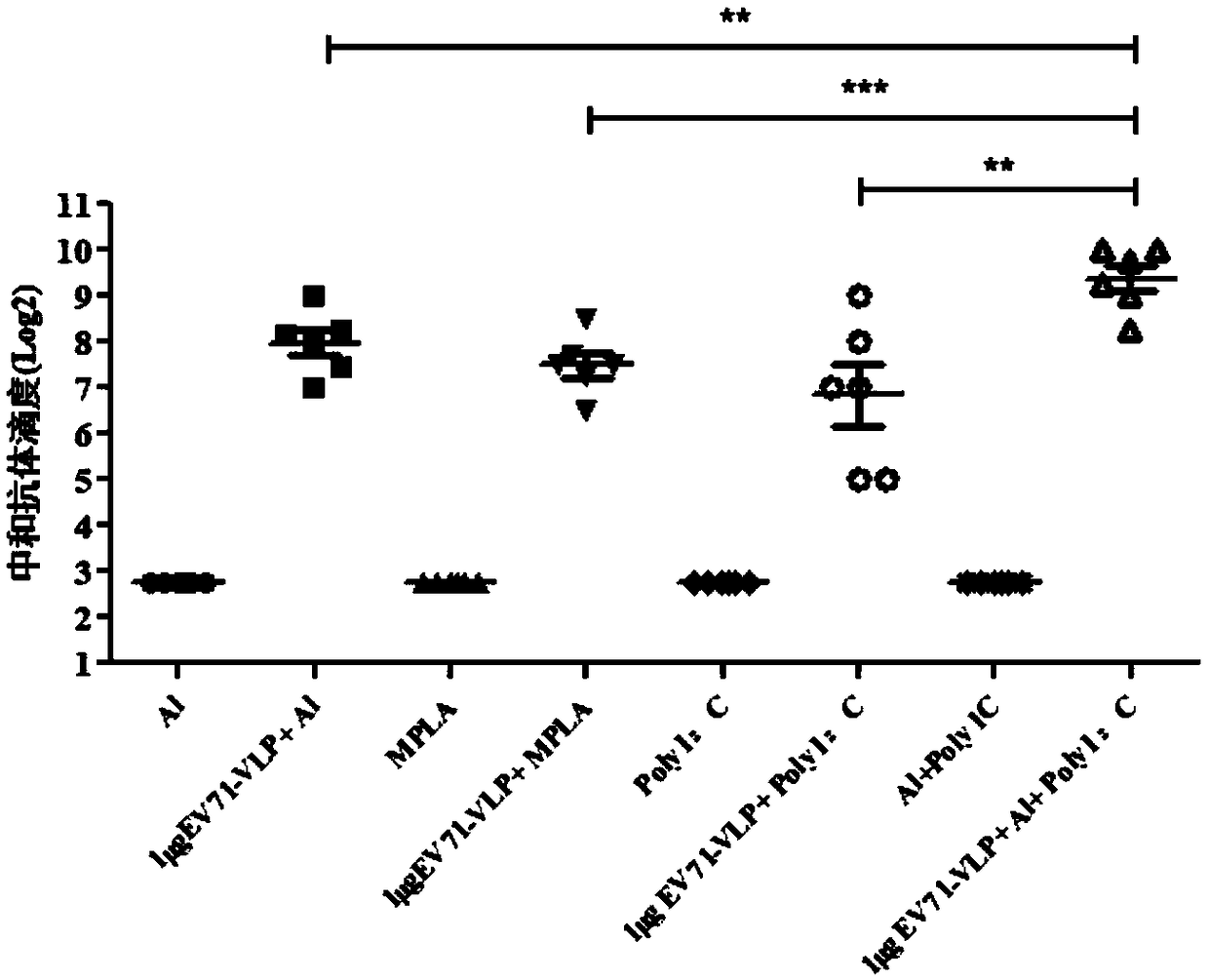
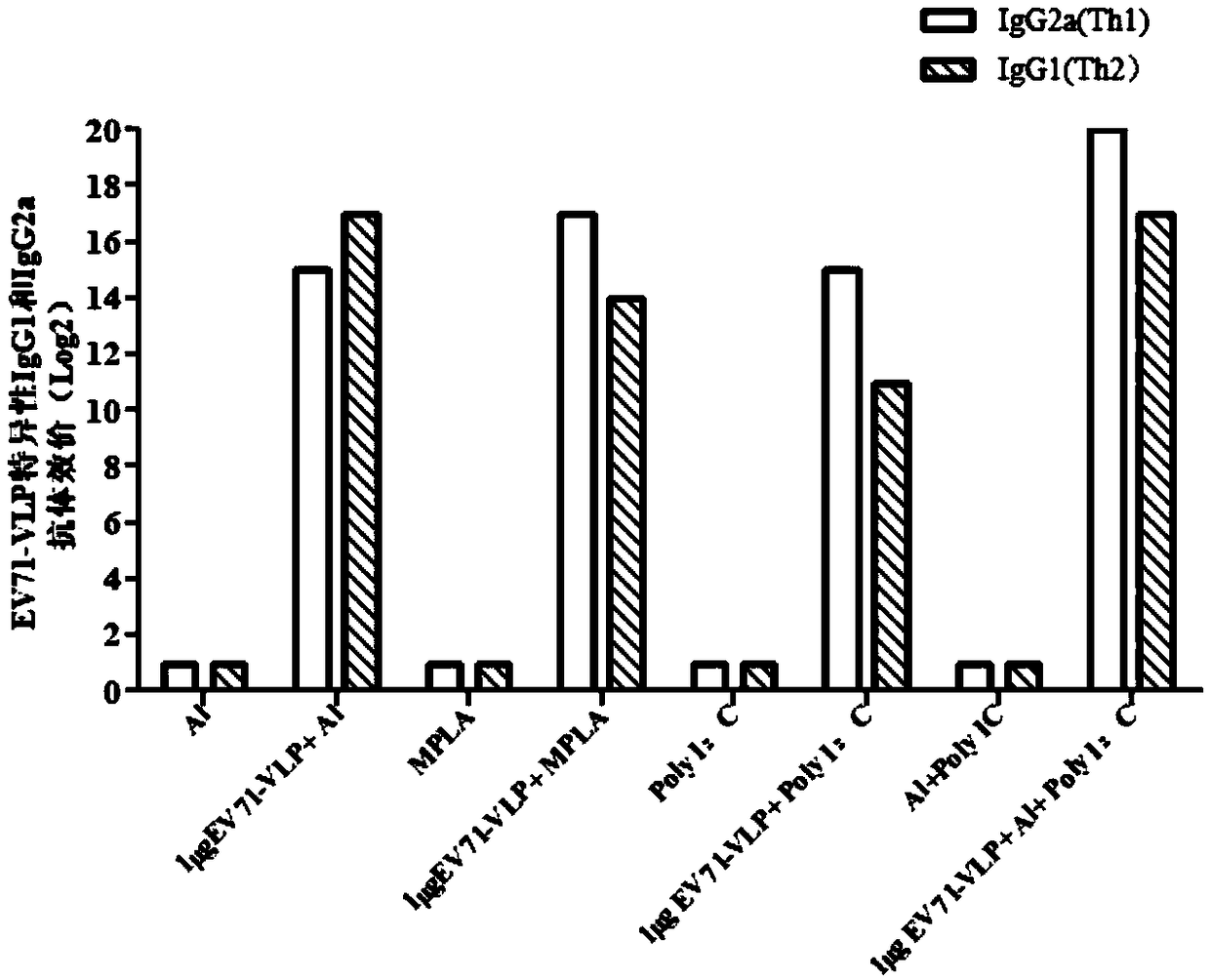
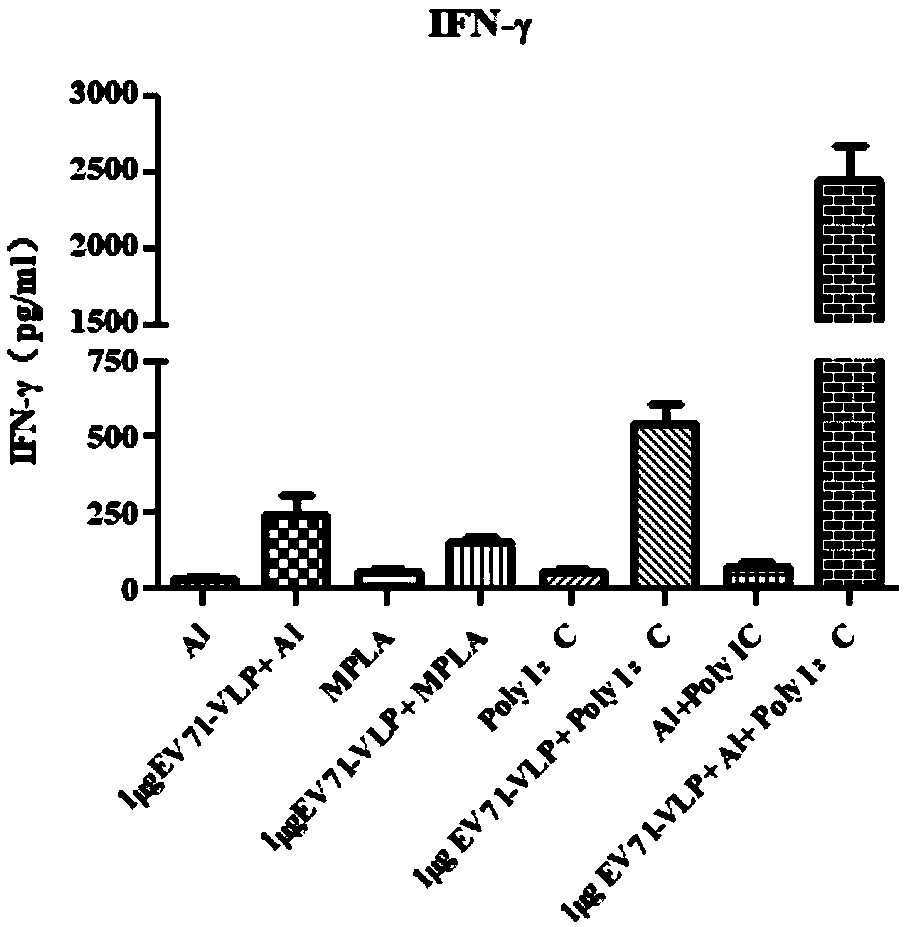
EV71 vaccine containing adjuvant combination
A technology of EV71 and EV71-VLP, applied in the field of biomedicine, can solve problems such as weak immunogenicity and poor immune response, and achieve the effect of reducing morbidity, severe disease and death cases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 A kind of EV71 vaccine containing adjuvant combination
[0033] Vaccine preparation method of the present invention
[0034] Reference patent: Preparation method of ZL201310697541.X to prepare EV71-VLP;
[0035] The content of PolyI:C (polyinosinic acid-polycytidylic acid) in each dose of vaccine is 50 μg, the content of EV71-VLP (EV71 virus-like particle) is 1 μg, and aluminum hydroxide adjuvant (the weight of aluminum element is 100 μg) . The EV71 antigen is EV71-VLP expressed using the baculovirus vector system.
Embodiment 2
[0036] Embodiment 2 A kind of EV71 vaccine containing adjuvant combination
[0037] Vaccine preparation method of the present invention
[0038] Reference patent: Preparation method of ZL201310697541.X to prepare EV71-VLP;
[0039] The content of PolyI:C (polyinosinic acid-polycytidylic acid) in each dose of vaccine is 20 μg, the content of EV71-VLP (EV71 virus-like particle) is 1 μg, and aluminum hydroxide adjuvant (the weight of aluminum element is 50 μg) . The EV71 antigen is EV71-VLP expressed using the baculovirus vector system.
Embodiment 3
[0040] Embodiment 3 A kind of EV71 vaccine containing adjuvant combination
[0041] Vaccine preparation method of the present invention
[0042] Reference patent: Preparation method of ZL201310697541.X to prepare EV71-VLP;
[0043] The content of PolyI:C (polyinosinic acid-polycytidylic acid) in each dose of vaccine is 80 μg, the content of EV71-VLP (EV71 virus-like particle) is 1 μg, and aluminum hydroxide adjuvant (the weight of aluminum element is 150 μg) . The EV71 antigen is EV71-VLP expressed using the baculovirus vector system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com