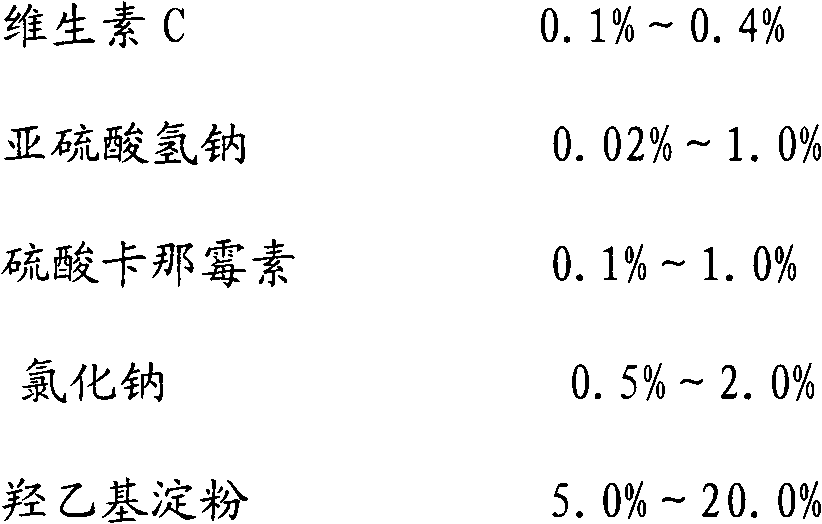
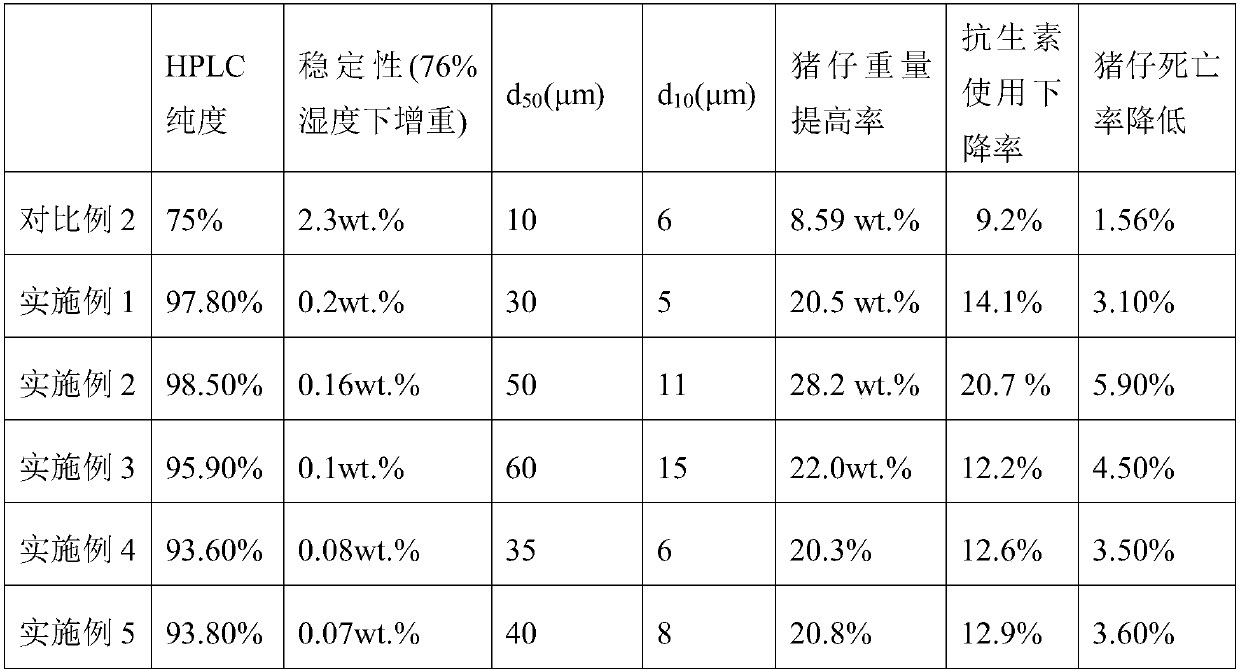
Patents
Literature
83 results about "Cytidylic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of polyinosinic acid-polycytidylic acid lyophilized powder injection
InactiveCN102988303ASlow down the rate of oxidative degradationLong validity periodOrganic active ingredientsPowder deliverySide effectOrganic chemistry
The invention discloses a preparation method of a polyinosinic acid-polycytidylic acid lyophilized powder injection. The method comprises the preparation of a polyinosinic acid-polycytidylic acid solution and a preparation of a lyophilized powder injection thereof. According to the invention, proper amounts of polyinosinic acid and polycytidylic acid are respectively dissolved by using normal saline; the mixtures are mixed and stirred, such that the polyinosinic acid-polycytidylic acid solution is prepared; a lyophilization additive is added and well mixed; and filtering, sub-packaging, and lyophilization are carried out. The prepared injection is suitable for frost preservation under a temperature below 0 DEG C. Therefore, expiration date is postponed, oxidative degradation speed is reduced, toxic and side effect are reduced, and stability is improved.
Owner:天津泽世德生物医药有限公司
Weaned piglet nucleotide feed additive
InactiveCN102987169AImprove antioxidant capacityEnhance immune functionAnimal feeding stuffBiotechnologyGuanosine monophosphate
The invention discloses a weaned piglet nucleotide feed additive. The weaned piglet nucleotide feed additive comprises, by weight, 0.8 to 1.3 of adenosine monophosphate 5'-AMP, 0.3 to 0.8 of cytidine monophosphate 5'-CMP, 1.1 to 1.5 of guanosine monophosphate 5'-GMP and 0.7 to 1.4 of uridine monophosphate 5'UMP. The invention further discloses a preparation method and an application of the feed additive. According to the weaned piglet nucleotide feed additive, stress reaction of weaned piglets can be effectively relieved, the piglet grazing rate and the feed utilization efficiency are improved, the oxidation resistance and the immune function of weaned piglets are improved apparently, the organic resistance is enhanced, and accordingly, important significances are provided for modern scientific, intensive and large-scale pig production.
Owner:NANJING BIOTOGETHER
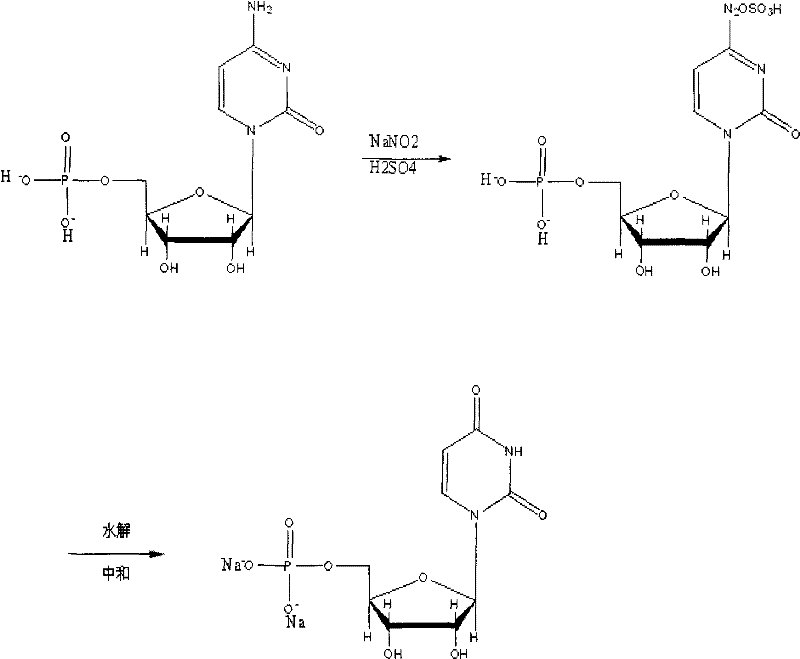
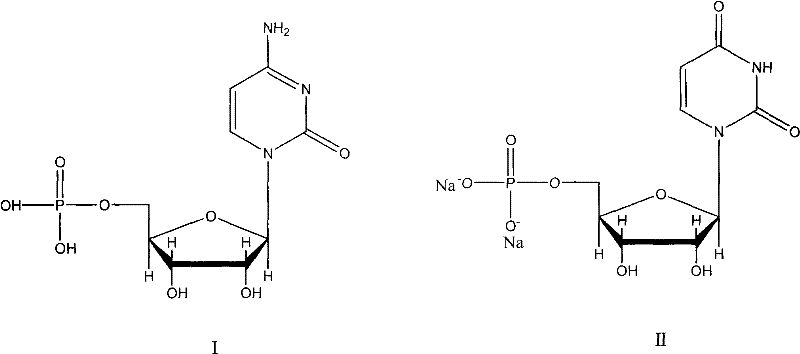
New method for synthesizing uridylic acid disodium
ActiveCN102250177AThe synthesis process is simpleImprove stabilitySugar derivativesSugar derivatives preparationOrganic acidWater vapor
The invention discloses a new method for synthesizing uridylic acid disodium. The method comprises the following steps: adding cytidylic acid and sodium nitrite in deionized water, and dropwise adding inorganic or organic acid at the constant temperature of minus 5 DEG C-0 DEG C; stirring the solution and heating to 10-60 DGE C, reacting for 1-12 hours, and introducing steam for steam distillation after the reaction is finished so as to obtain a uridylic acid solution; regulating the pH of the uridylic acid solution to 7.0-8.5 with alkali, distilling at reduced pressure until a liquid is not distilled out; adding 95% ethanol the weight of which is 3-12 times that of cytidylic acid, heating and refluxing for 0.5-6 hours under the condition of stirring, crystallizing, and filtering so as to obtain crude uridylic acid disodium; and dissolving crude uridylic acid disodium in a proper amount of water, recrystallizing with ethanol the weight of which is 1-3.5 times that of the crude uridylic acid disodium, filtering and drying so as to obtain the uridylic acid disodium product. By using the method, the yield of uridylic acid disodium is above 92.75%, the purity of uridylic acid disodium by detection of high performance liquid chromatography (HPLC) is more than 99.50%, and the purity of uridylic acid disodium by detection of ultraviolet (UV) light is above 98.50%. The method has the advantages of simple and reasonable process, good product quality stability, low production cost and the like, and is easy to be applied to massively production.
Owner:宋道淮
Ubelin manufacturing technique
InactiveCN101130797ASolve pollutionHigh activityMicroorganism based processesFermentationUltrafiltrationCholine Phosphate
The present invention relates to a production process of citicoline. It is characterized by that said production process includes the following steps: firstly, adding water and glucose into a reaction tank, then adding yeast, making fermentation, after fermentation adding inorganic salt and choline phosphate, the adding cytidine monophosphate solution, an adding cane sugar, heating, then quickly-cooling by using water, pressing reaction liquid, washing by using water, removing salt from obtained clear liquor; then pressing said clear liquor and making said clear liquor be fed into a carbon column, rinsing said carbon column by using pure water, then using ethyl alcohol solution to make elution, collecting citicoline sodium; then vaporizing and concentrating eluent, diluting concentrate and making it be fed into a macroporous ion exchange resin column, then washing said column by using water and making elution, concentrating eluent, heating the collected citicoline sodium solution, removing impurity by utilizing ultrafiltration device, decolouring, adding ethyl alcohol and stirring them, storing the above-mentioned material in a refrigeration house and staying overnight; then making crystal solution undergo the processes of centrifugal separation, vacuum drying, bagging, weighing, sealing and dry-storage.
Owner:张剑
Mixed nucleotide which comes from yeast and contains uridylic acid and preparing method and application thereof
The invention relates to the industrial field of animal feed, in particular to mixed nucleotide which comes from yeast and contains uridylic acid and a preparing method and application thereof. The mixed nucleotide contains, by weight, 43-80 parts of cytidine monophosphate, 116-200 parts of adenylate, 95-150 parts of guanylic acid, 400-800 parts of uridylic acid and 1 part of inosinic acid. The invention further provides a yeast autolysate containing the mixed nucleotide, wherein the weight percentage of the mixed nucleotide in the yeast autolysate is 1.5-2%. By the adoption of the yeast autolysate, the growing speed of piglets can be effectively increased, productivity and feed conversion rate are high, average daily gain of piglets is increased by 9.8-17.4%, and application prospects are broad.
Owner:ANGELYEAST CO LTD
A strain of Issakia orientalis and its whole cell transformation method for producing citicoline
InactiveCN102286386AEfficient preparationIncrease regeneration rateFungiMicroorganism based processesCholine PhosphateManganese
The invention relates to Issatchenkia orientalis and a method for producing citicoline by whole cell conversion of Issatchenkia orientalis, belonging to the technical field of biological pharmacy. In the method provided by the invention, the whole cells of Issatchenkia orientalis Z1, namely CCTCC (China Center for Type Culture Collection) NO: M2011272 are utilized to prepare citicoline, choline phosphate and 5'-cytidylic acid are used as substrates, glucose is used as an energy donor, and the ATP (adenosine triphosphate) regeneration efficiency is improved by adding inorganic ions; glucose is used as the energy donor, so that the energy requirement of the strain is provided and ATP is provided for a citicoline synthesis enzyme system; one or more of potassium ion, magnesium ion and manganese ion are added to change the metabolism flow direction and improve the ATP regeneration rate, so that the ATP regeneration rate is matched with the rate of the citicoline enzyme synthesis system and the high-efficiency preparation of citicoline is achieved; and the whole cells of Issatchenkia orientalis are used, and toluene is added to an aqueous solution during the preparation process so as to improve the cell permeability, so that the rate of the citicoline enzyme synthesis system is improved.
Owner:江苏华晟知识产权运营有限公司
Method of preparing cytidine disodium triphosphate and application
The invention relates to a manufacturing method of disodium cytidine triphosphate, which comprises the following steps: proceeding enzymatic conversation with CMP material; proceeding ion exchange column chromatography; separating and purifying. The enzymatic conversation reaction principle is that: brewers' yeast gets the needed enzyme liquid of the enzymatic conversation reaction; brewers' yeast produces a plurality of biological enzyme as sugar glycolysis enzyme and phosphorylase with high sugar glycolysis ability; the biological energy produced by glycolysis glucose with sugar glycolysis enzyme is transferred to cytidine monophosphate by ATP; cytidine monophosphate and phosphate radical combines to form cytidine triphosphate with the effect of phosphorylase. The method shapes low cost, simple operation, high production, high product purity by optimization, which comprises the following steps: getting enzyme liquid; proceeding enzymatic conversation by adding the main material cytidine monophosphate, monobasic potassium phosphate of proper quantity and glucose; getting product by deposition, ion exchange column chromatography, segregation, purification, refining and vacuum drying.
Owner:BEIJING SL PHARMA
Milk powder of immune formula
The invention provides a milk powder of an immune formula. Each 100g of milk powder comprises the following components: 20-50g of a raw milk extract, 30-55g of demineralized whey powder, 5-20g of vegetable oil, 0.5-6g of probiotics, 20-100mg of inositol, 10-60mg of taurine, 8-50mg of L-carnitine and / or salt of L-carnitine, 50-150mg of choline matters and 15-45mg of nucleotide, wherein a weight ratio of five nucleotides including guanosine 5'-monophosphate disodium salt, cytidine 5'-monophosphate disodium salt, uridine-5'-monophosphate disodium salt, adenosine 5'-monophosphate disodium salt and guanosine 5'-monophosphate disodium salt is 1 to (0.1-10) to (1-5) to (0.1-5) to (0.1-3). The milk powder is relatively reasonable in formula ratio, and provides relatively complete nutrients and good absorption for infants, and the immunity of the infants is completely improved; meanwhile, components of maltodextrin, saccharose or white granulated sugar, essence, a pigment, an emulsifying agent and the like are not added, and thus the health of the infants is relatively facilitated.
Owner:北安宜品努卡乳业有限公司
Recombinant escherichia coli for high yield of cytidine monophosphate and application of recombinant escherichia coli
PendingCN111269870AMeet the requirements of large-scale industrial productionReduce purificationBacteriaTransferasesEscherichia coliEnzyme Gene
The invention discloses recombinant escherichia coli for high yield of cytidine monophosphate and application of the recombinant escherichia coli. According to the invention, cytidine kinase gene is obtained by cloning from a cytidine monophosphate production strain; crude enzyme liquid obtained by crushing recombinant bacteria has good catalytic activity and stability; cytidine, adenosine triphosphate (ATP) and Mg < 2 + > are added, the reaction system is simple, the conditions are mild, the period is short, byproducts are fewew, the method is clean and free of pollution, the application is asimple, rapid and efficient production way, and the conversion rate of substrate cytidine and ATP reaches 85% or above.
Owner:NANJING UNIV OF TECH
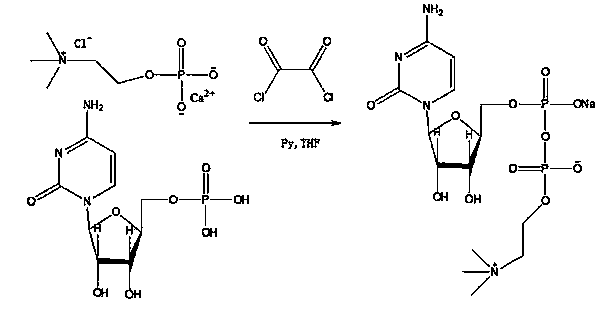
Method for preparing citicoline sodium by utilizing oxalyl chloride
ActiveCN104004040ALow costGood atom economySugar derivativesSugar derivatives preparationBenzeneCalcium biphosphate
The invention discloses a method for preparing citicoline sodium by utilizing oxalyl chloride. The method is characterized by comprising the following steps of: by taking choline chloride calcium phosphate (P-choline) as a raw material, dissolving in organic amine not containing reactive hydrogen, adding oxalyl chloride for reacting for 0.5-2 hours after adding benzene for removing water in an azeotropic manner, and then, adding cytidylic acid (5'-CMP) for reacting.
Owner:回音必集团抚州制药有限公司
Nucleotide feed additive for tilapia
ActiveCN103005220ASimple production processNo pollution in the processAnimal feeding stuffBiotechnologyFeed additive
The invention discloses a nucleotide feed additive for tilapia. The nucleotide feed additive is characterized in that nucleotide comprises adenylic acid 5'-AMP, cytidylic acid 5'-CMP, guanylic acid 5'-GMP and uridylic acid 5'-UMP in a weight ratio of (0.9-1.5):(0.7-1.2):(1.1-2.0):(0.8-1.5). The nucleotide feed additive has the beneficial effects that the nucleotide feed additive can obviously increase the length of the intestinal tract of tilapia and improve the growth efficiency, feed utilization rate and environment tolerance of tilapia, and at the same time, the non-specific immunity of tilapia is greatly enhanced by adding nucleotide, which have profound significance for the present intensive culture industry of intensive tilapia.
Owner:NANJING BIOTOGETHER
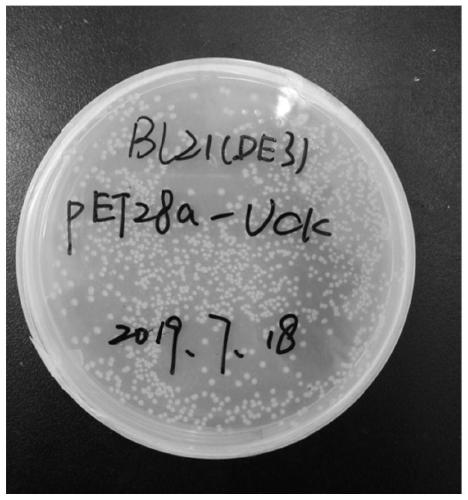
Method for knocking out cytidine deaminase (cdd) gene in escherichia coli by utilizing CRISPR-Cas9 technology and application
InactiveCN111321101ASimple way to knock out E. coli genesSimple methodBacteriaHydrolasesEscherichia coliCytidine Deaminase Gene
The invention discloses a method for knocking out a cytidine deaminase (cdd) gene in escherichia coli by utilizing a CRISPR-Cas9 technology and application. The method comprises the following steps: 1, making escherichia coli BL21 (DE3) competence containing a Cas9 plasmid; 2, designing and synthesizing a mutant sgRNA; 3, constructing Donor DNA; 4, transforming the sgRNA plasmid and the Donor DNAinto the escherichia coli competence carrying the Cas9 plasmid by employing an electro transformation method, and knocking out the cdd gene; and 5, introducing a target plasmid pET28a-UCK into a knock-out strain to produce cytidylic acid. The method has the advantages that operation is simple, the knockout success rate of the cdd gene is high, and the conversion rate of the knock-out strain on a substrate is improved, and is suitable for industrial production of the cytidine acid and the like. Compared with producing the cytidylic acid without strain knockout, producing the cytidylic acid withstrain knockout improves the conversion rate of the substrate cytidine and adenosine triphosphate (ATP) by 15%, and the conversion rate reaches 99%.
Owner:NANJING UNIV OF TECH
Nucleotide mixture crystalline powder and preparation method thereof
The invention discloses nucleotide mixture crystalline powder and a preparation method thereof. The preparation method comprises the following steps of performing solid-liquid separation on ribonucleic acid enzymatic hydrolysate, decoloring the enzymatic hydrolysate, regulating the pH of decolored enzymatic hydrolysate to 6.5-10.0, performing concentration, performing crystallization, performing filtration, and performing drying so as to obtain the nucleotide mixture crystalline powder containing uridylic acid, guanylic acid, cytidylic acid and adenylic acid. The nucleotide mixture crystallinepowder provided by the invention is high in purity, good in stability and free from moisture absorption, has favorable granularity and mobility, and can be widely applied to feed trade. The preparation method provided by the invention is high in yield, simple to operate and low in cost, and is suitable for industrial production.
Owner:NANJING UNIV OF TECH
Partially reduced graphene oxide, preparation method and application thereof
ActiveCN109607517AStable in natureLarge specific surface areaGrapheneOn/in inorganic carrierAdenosineDipotassium guanylate
The invention discloses a preparation method of partially reduced graphene oxide, belonging to the application technical field of graphene oxide. Graphene oxide is dispersed in water, a reducing agentis added and reacted to obtain partially reduced graphene oxide; the reducing agent is one or more of components selected from adenylate, disodium adenylate, dipotassium adenylate, adenosine-5'-diphosphate dipotassium salt, cytidylic acid, cytidine-5'-monohposphate disodium salt, cytidine dipotassium, guanylic acid, disodium guanylate, dipotassium guanylate, uridylic acid, disodium uridine-5'-monophosphate, and dipotassium uridine-5'-monophosphate. Nucleotides have different types of R groups, and can interact with graphene oxide by various forces, such as hydrophobic force, electrostatic force and hydrogen bond force, so as to control the surface properties of graphene oxide.
Owner:NANJING UNIV OF TECH
Composition for treating and/or preventing hepatitis b virus infection and use thereof
PendingCN110944662AAvoid infectionReduce infectionOrganic active ingredientsPharmaceutical delivery mechanismHepatitis B virus core AntigenAntigen
A composition for treating and / or preventing Hepatitis B virus infection and Hepatitis B virus infection mediated diseases and the method thereof are provided. In some embodiments, the composition includes a polyriboinosinic acid-polyribocytidylic acid (PIC), at least one antibiotic or polyamide compound, at least one positive ion, and Hepatitis B virus surface antigen. In some embodiments, the composition includes PIC, at least one antibiotic or polyamide compound, at least one positive ion, Hepatitis B virus surface antigen and Hepatitis B virus core antigen. The present disclosure also relates to a method of treating and / or preventing Hepatitis B virus infection, particularly for treating chronic HBV infection.
Owner:YISHENG BIOPHARMA SINGAPORE
Method for preparing 5'-cytidylic acid from composite solvent and bind acid agent
InactiveCN102212096AImprove solubilityImprove dissolution rateSugar derivativesSugar derivatives preparationPhosphorylationSolvent
The invention relates to a method for preparing 5'-cytidylic acid from a composite solvent and a bind acid agent. The method comprises the following detailed steps of: (1) preparing the composite solvent which contains a sulfoxide solvent and alkyl three-low level phosphate; (2) adding a phosphorylation agent, namely at the temperature of -2 to 2 DEG C, dropwise adding the phosphorylation agent into the composite solvent to perform an intervention reaction, wherein the phosphorylation agent and a certain amount of water in the composite solvent react to generate a part of hydrate; (3) adding cytidine, namely gradually adding the cytidine at the temperature of 0 to 5 DEG C to perform reaction for 3 to 5 hours; and (4) adding the bind acid agent, namely adding the phosphorylation agent and the bind acid agent in a proportion of 100:0.8-100:0.6. The yield of the product obtained by the method is over 90 percent; the ultraviolet content of the generated 5'-cytidylic acid is over 98 percent; and the high-performance liquid chromatography (HPLC) content of the generated 5'-cytidylic acid is over 98 percent.
Owner:NANTONG SANE BIOLOGICAL
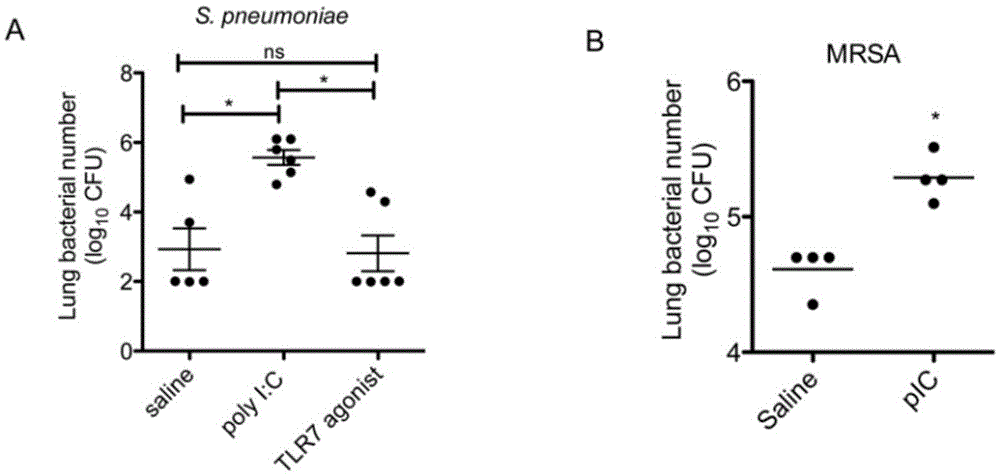
Application of poly inosine-cytidine, imiquimod and gardiquimod in constructing virus immunity mouse model
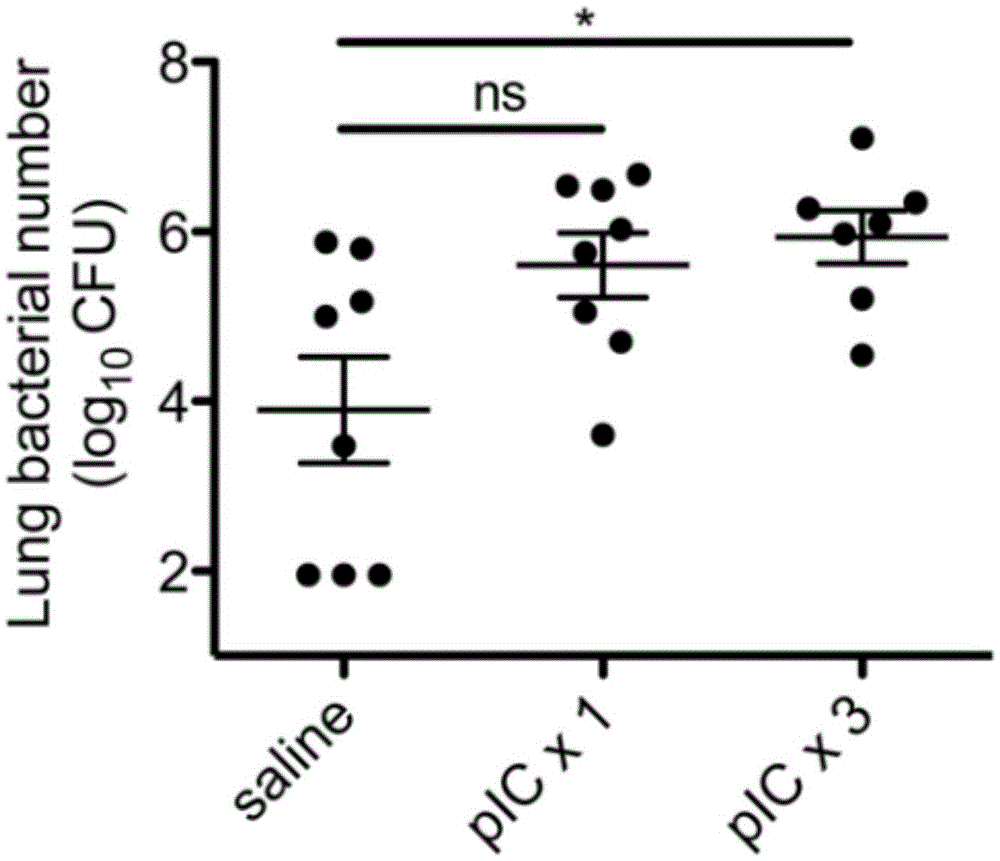
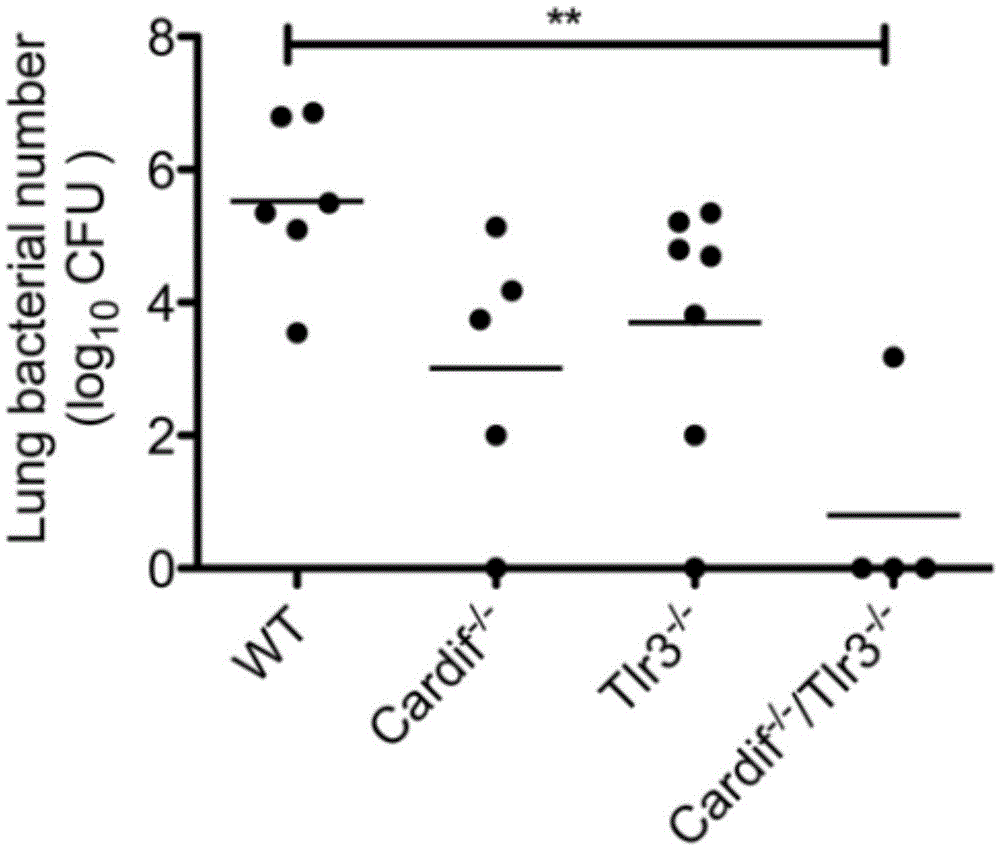
The invention relates to the field of biological medicine, in particular to an application of poly inosine-cytidine, imiquimod and gardiquimod in constructing a virus immunity mouse model. The inventor of the invention employs the mouse model and forms an antivirus immune state by using poly inosine-cytidine, imiquimod and gardiquimod; an experiment mouse administered with poly inosine-cytidine is found to appear lung bacterium clearance impairment; in addition, poly inosine-cytidine is found to be capable of mediating IFNI expression; and finally, IFNI can induce a defense mechanism of a lung against gram positive pathogenic bacteria.
Owner:宁波美丽人生医药生物科技发展有限公司
Pharmaceutical composition of dsRNA and astragalus polysaccharide and application thereof
InactiveCN101703518AImprove immunityEasy to solveOrganic active ingredientsAntiviralsBiotechnologyAstragalus polysaccharide
The invention relates to a pharmaceutical composition of dsRNA and astragalus polysaccharide and application thereof, in particular to the pharmaceutical composition applied to viral infection prevention and treatment of cultured animals. The pharmaceutical composition is formed by combining the astragalus polysaccharide and the dsRNA according to a certain proportion, wherein the astragalus polysaccharide has the content range of 20-50mg / ml; the dsRNA (polyinosinic acid) has the content range of 1-5mg / ml and the molecular weight range of 200-500bp; and the main components of the astragalus polysaccharide and the dsRNA are processed by matching liquid, filtering, filling and encapsulating as well as sterilizing according to water injection production technology, and the pharmaceutical composition can be obtained after being checked to be qualified. A certain amount of polyinosinic cytidylic acid is added into water for injection and dissolved under the condition of 20-60 DEG C; then, equimolar polycytidylic acid is added into the solution to be dissolved; heat preservation and pairing are carried out for 15-50min; and right amount of astragalus polysaccharide is added, evenly mixed and treated by filtering, filling and encapsulating as well as sterilizing. Therefore, the invention is applicable to improving the immunity of livestock, poultry and aquatic animals, and improves the prevention and treatment for virus diseases.
Owner:青岛汉河动植物药业有限公司
Method for efficiently producing DC-CIK cells through induction of polyinosinic: polycytidylic acid copolymer
PendingCN111733129AEnhance tumor killing activityEasy to killCulture processBlood/immune system cellsCancer cellSpecific immunity
The invention relates to the technical field of immunization, in particular to a polyinosinic: polycytidylic acid copolymer (Poly I: C). The polyinosinic: polycytidylic acid copolymer is a ligand of atype-3 Toll-like receptor in an animal body, can mediate a series of immune reactions of the body after TLR-3 is activated, and has a good promoting effect on specific immunity and non-specific immunity of the body. Compared with a control group, DC-CIK induced by the polyinosinic: polycytidylic acid copolymer has higher proliferation, high differentiation (especially CD3+CD4-CD8+, CTL) and hightumor killing activity. The autologous efficient DC-CIK induced by the polyinosinic: polycytidylic acid copolymer and other immune cells are applied to treatment of clinical tumor patients. The polyinosinic: polycytidylic acid copolymer aims to recover or improve the immune function of the tumor patients, and the immune system of the body is improved to kill and inhibit proliferation of tumor cells. The tumor cell load is reduced, tiny residual lesions are removed, or the proliferation mode of residual tumor / cancer cells is obviously inhibited, factors such as relapse and metastasis are eliminated, the cure possibility is increased, the survival time is prolonged, and the life quality is improved. Therefore, the purpose of treating tumors / cancers is achieved.
Owner:成都源泉生物科技有限公司 +1
Method for separating and purifying citicoline in issatchenkia orientalis biotransformation liquid
ActiveCN103819522ALow costHigh yieldSugar derivativesSugar derivatives preparationIon-exchange resinDrug biotransformation
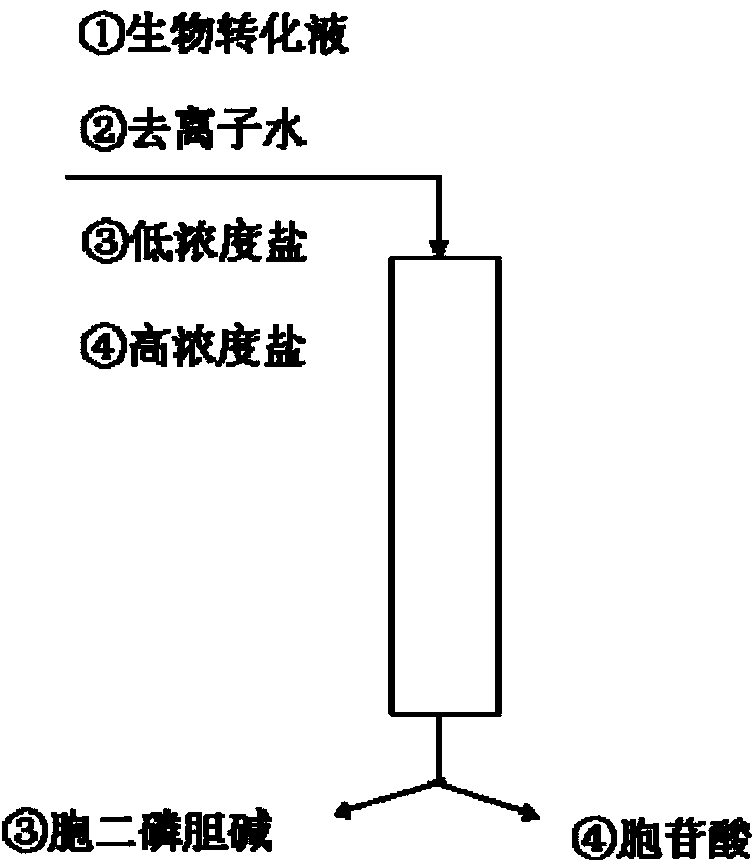
The invention discloses a method for separating and purifying citicoline in an issatchenkia orientalis biotransformation liquid. The method comprises the following steps: the issatchenkia orientalis CDP-c biotransformation liquid is passed through an anion exchange resin column, deionized water is used for leaching an unadsorbed substance, a salt solution of 0.01-0.50M is employed for eluting CDP-C and the salting liquid of 0.10-1.00M is employed for eluting cytidylic acid, so that CDP-c with high purity (greater than 99%) and high yield (greater than 88%) can be obtained by one time, and the cytidylic acid without complete reaction can be recovered. The method for separating and purifying citicoline in the issatchenkia orientalis biotransformation liquid greatly simplify a separating process, the operation is simple, the cost is low, and the environment is protected, and is adapted to large-scale industrial production.
Owner:无锡泰仑达化机设备有限公司
Polynucleotide microcapsule for treating diseases of livestock and poultry and preparation method thereof
InactiveCN101474165AProlong the action timeNo side effectsOrganic active ingredientsSugar derivativesDiseaseFiber
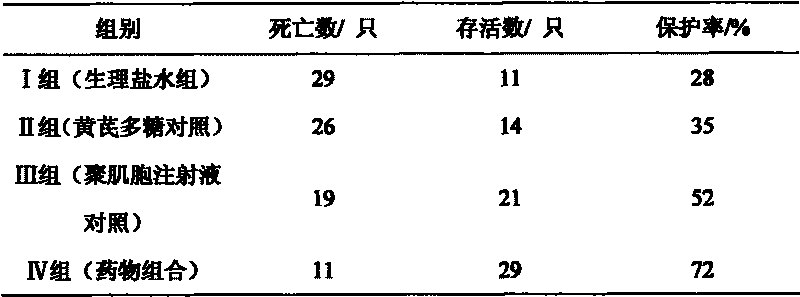
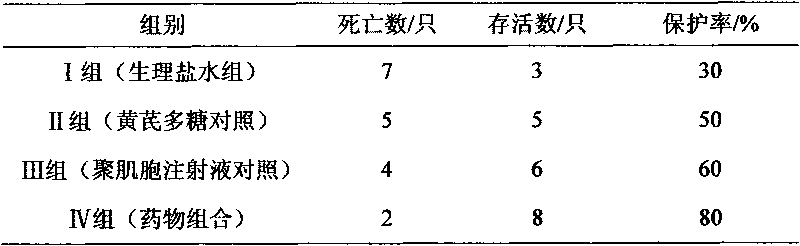
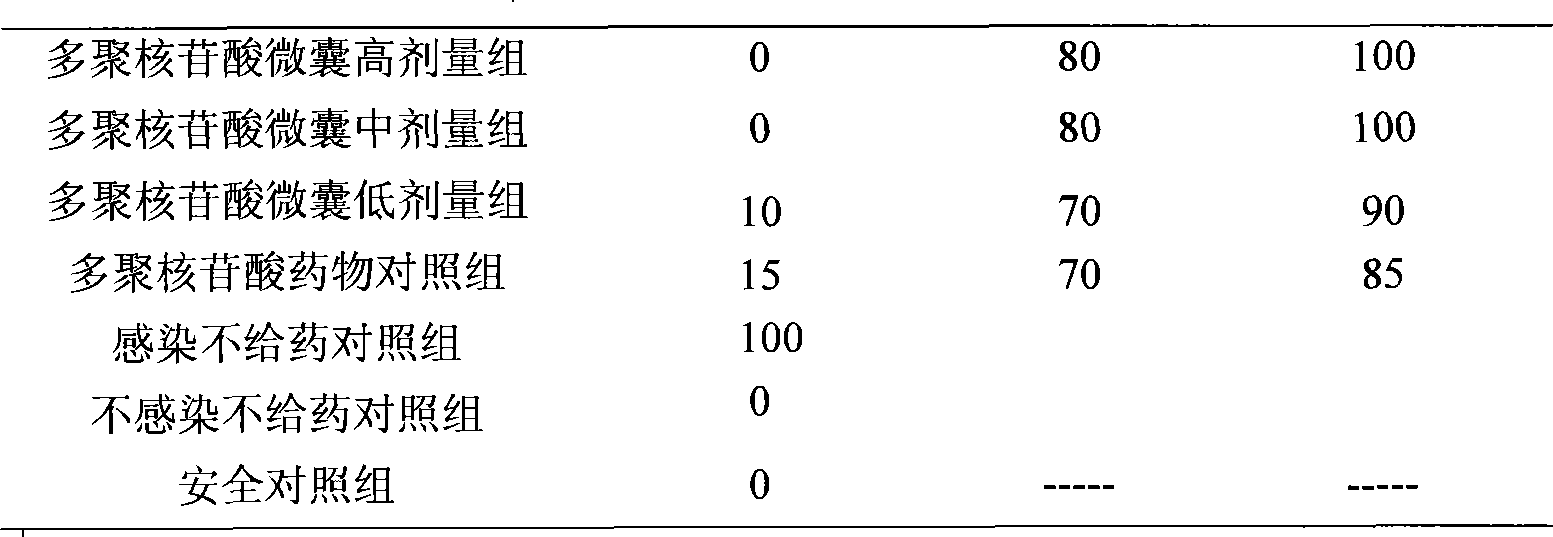
The invention relates to a polynucleotide microcapsule for treating animal disease and a preparation method thereof, belonging to the technical field of veterinary medicament preparation. Polyinosinic acid and polycytidylic acid are weighted and are added into preheated sodium chloride-phosphate buffer solution, and polynucleotide solution is prepared; the polynucleotide solution is added into ethyl cellulose to prepare soft wood and dry powder is prepared; the dry powder and magnesium stearate are added into ice-bath acetone to obtained a mixture, the mixture is stirred and slowly added into whiteruss containing 2%Span-80 surface active agent; and polynucleotide microcapsule is obtained by a method of in-liquid drying. The invention effectively prolongs the acting time of medicament within human body, decrease the applied time of the medicament and achieves the aims of long acting and sustained release. Experiments prove that the dead rate of the high dose group or the medium dose group of the polynucleotide microcapsule is obviously lower than that of an infectious control group. The cure rate is high, both are 80%, and the effective rate of the two groups are 100%. Experiments prove that the medicament of the invention is safe.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
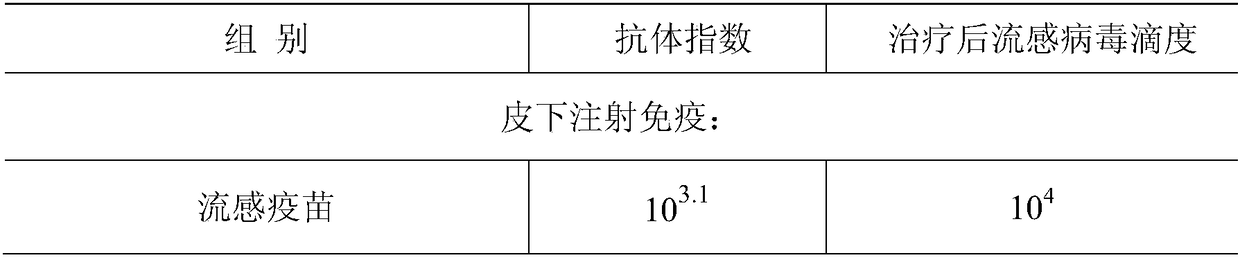
Mucosal immunity preparation capable of resisting infection and tumors
ActiveCN109125264AExtended half-lifeImprove effectivenessAntibacterial agentsPowder deliveryDiseaseHalf-life
The invention relates to the field of immunology, and particularly discloses a mucosal immunity preparation capable of resisting infection and tumors. The effective component of the preparation is a mucosal immunity substance which is formed by combining polyinosinic acid-polycytidylic acid, a non-antibiotic amino compound, metal positive ions, and PEG and / or PEI and / or PLGA and / or positive ion polymers through hydrogen bonds and other chemical bonds. The preparation is a spray or an aerosol in dosage form, but preferentially, the preparation does not contain ingredients utilized by common sprays or ingredients utilized by aerosols, and propellants. The mucosal immunity preparation can achieve the slow-release function partially or wholly, the half-life period of the mucosal immunity preparation is prolonged, the availability and effectiveness of drugs are improved, and the compliance of a patient is improved. The mucosal immunity preparation can promote mucosal immunity of the human body by means of the mucosal immunity, and then promote activation and proliferation, including whole-body nonspecific immunity, humoral immunity and cellular immunity, of all immune cells instead of only acting on the local part of a disease wound, and thus the preventing and treating effects of resisting infection and tumors are achieved.
Owner:林海祥 +3
Synthetic method of citicoline sodium
InactiveCN111808899AAvoid disadvantages that need to be dealt withEnzyme source stabilityTransferasesFermentationCholine PhosphateCiticoline sodium
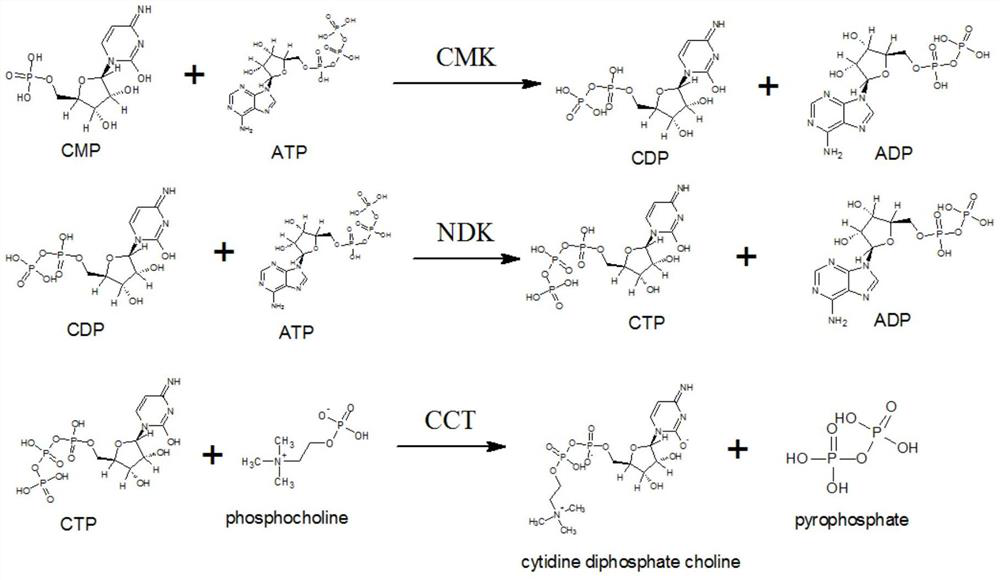
The invention discloses a synthetic method of citicoline sodium. The synthetic method comprises the following steps: adding 5'-cytidylic acid, calcium phosphorylcholine chloride, ATP, an aqueous magnesium chloride solution and a Tris-HCl buffer solution into a reaction vessel, and carrying out uniform mixing; then, adjusting a pH value to 6.0-8.0, adding CMP phosphokinase, nucleoside diphosphate kinase and choline phosphate-cytidyltransferase, and carrying out a stirring reaction for 4-10 hours at a temperature of 25-50 DEG C; and conducting high-speed centrifugation, and taking a supernatantto obtain citicoline sodium. Compared with the traditional citicoline sodium synthesis process, the synthetic method provided by the invention is environment-friendly in a synthesis route, stable in yield and is beneficial for reducing energy consumption and protecting the environment, so the synthetic method is suitable for large-scale industrial production.
Owner:ENZYMASTER NINGBO BIO ENG CO LTD
Pharmaceutical composition comprising particles comprising a complex of a double-stranded polyribonucleotide and a polyalkyleneimine
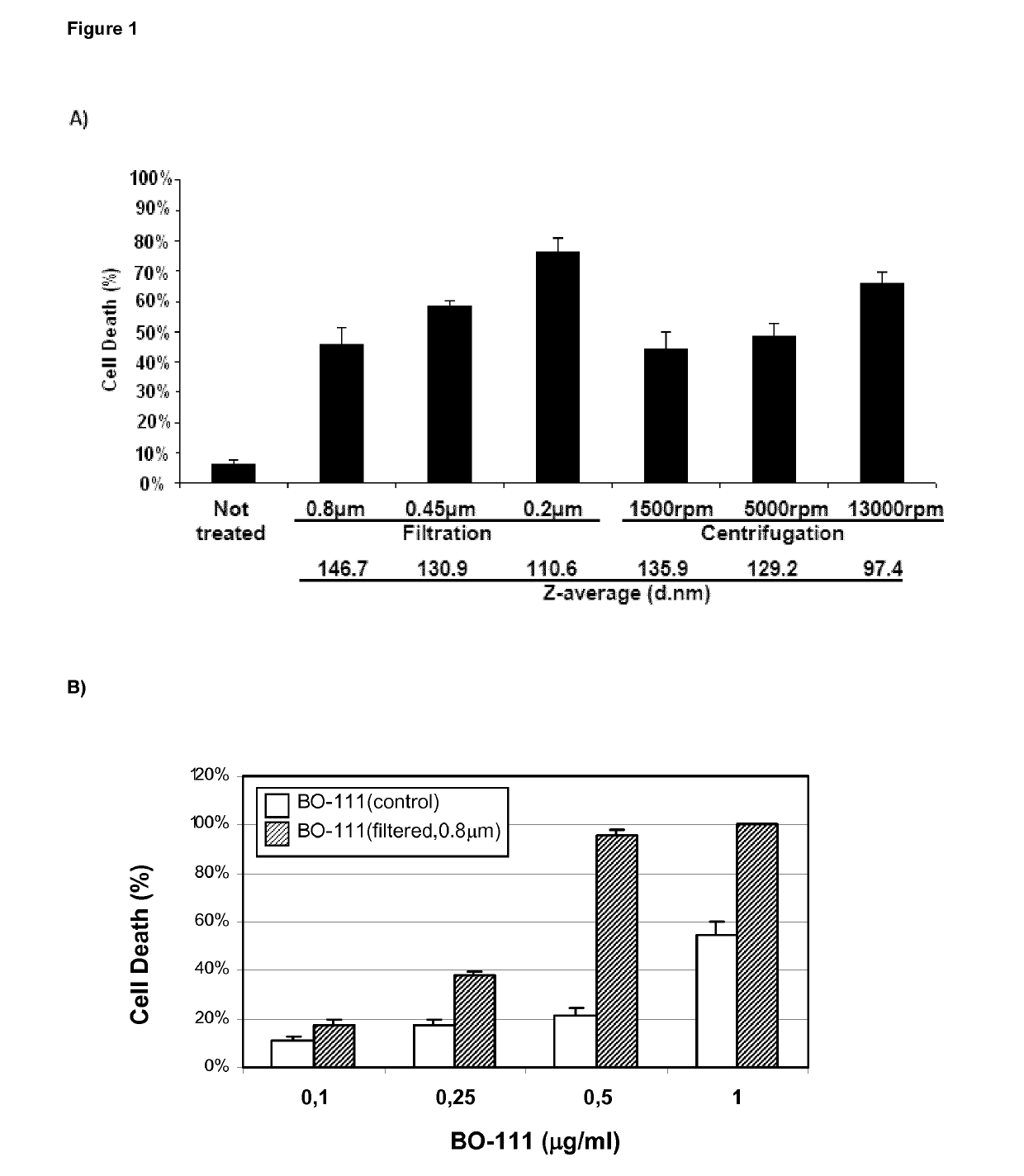
The present invention relates to compositions comprising particles, each of said particles comprising a complex of at least one double-stranded polyribonucleotide, such as polyinosinic-polycytidylic acid [poly(I:C)], and at least one linear polyalkyleneimine. The particles are also characterized by their monomodal diameter distribution and z-average diameter within specific ranges. The present invention additionally relates to use of said compositions as medicaments, in particular for the treatment of a cell growth disorder characterized by abnormal growth of human or animal cells, as well as to processes for the preparation of said compositions.
Owner:HIGHLIGHT THERAPEUTICS SL
Method for evaluating destroy degree on nucleotide of determination method and production technology of free nucleic acid hydrolysate in protein product
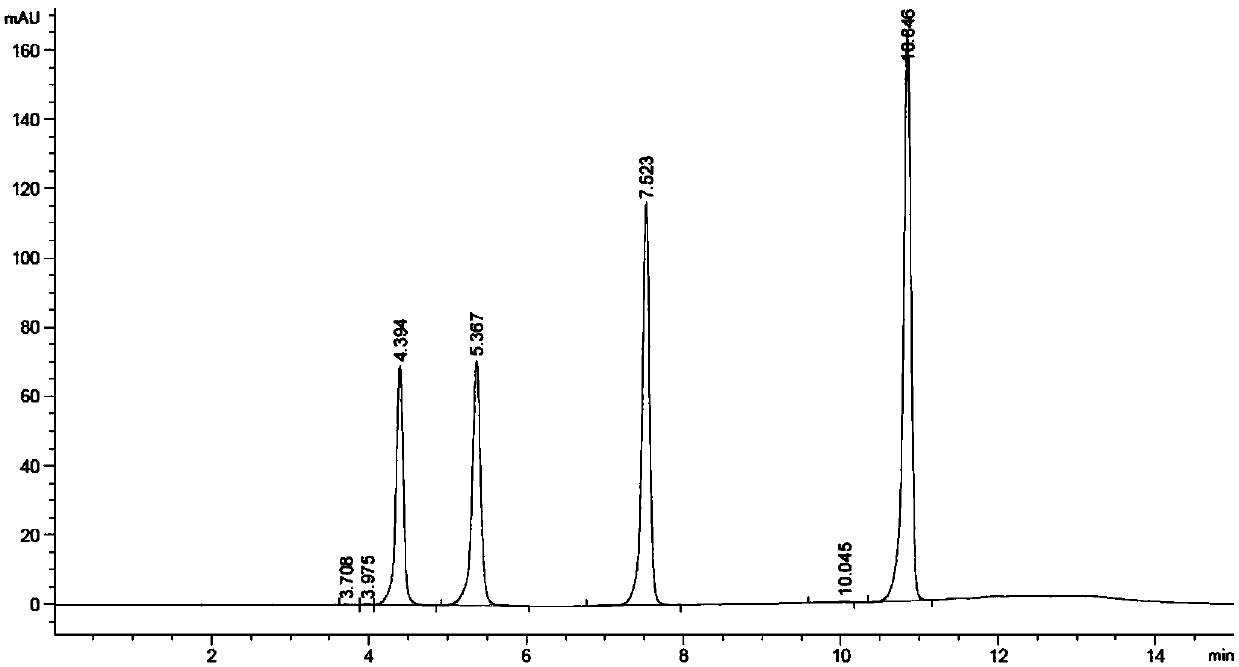
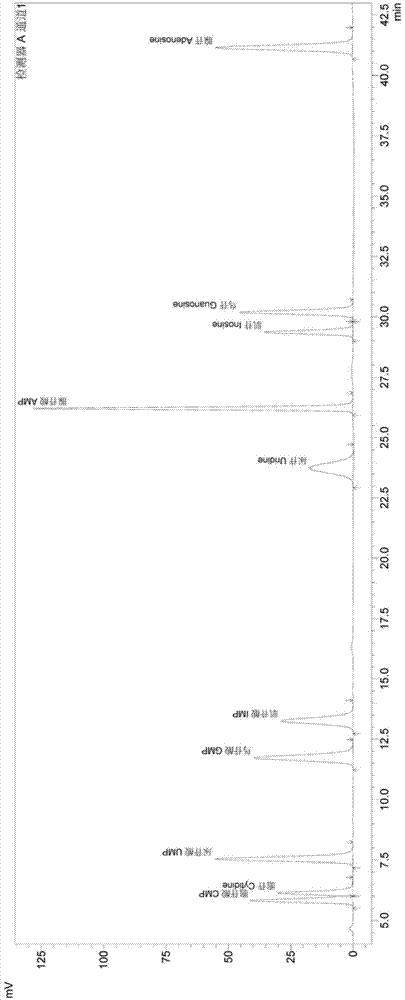
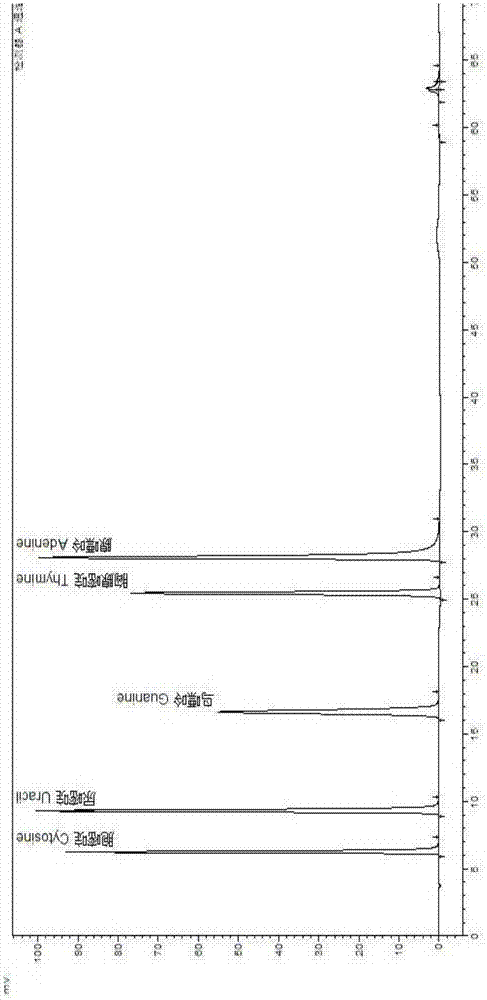
The invention discloses a method for determining a free nucleic acid hydrolysate in protein products. The method comprise the following steps: (1) respectively preparing a nucleotide mixed standard solution and a bases mixed standard solution; (2) preparing a solution of a sample to be tested, and using the nucleotide mixed standard solution and the base mixed standard solution described in step 1 as the reference solution, and employing high performance liquid chromatography through washing peak area method comparison to obtain the content of free nucleotides and free base in the sample. According to the invention, high performance liquid chromatography is used for determining 5 kinds of bases in a single-cell protein raw material (cytosine, uracil, guanine, thymine and adenine), 5 kinds of nucleotides (cytidine monophosphate, uridylic acid, guanylic acid, inosinic acid and adenylic acid), and 5 kinds of nucleosides (cytidine, uridine, inosine, guanosine and adenosine); and then the destroy degree on nucleotide of the production process is determined according to t the relationships between the bases and nucleotides, and between the bases and nucleosides.
Owner:江苏征泰饲料有限公司
Low-protein feed for improving growth performance of litopenaeus vannamei and application thereof
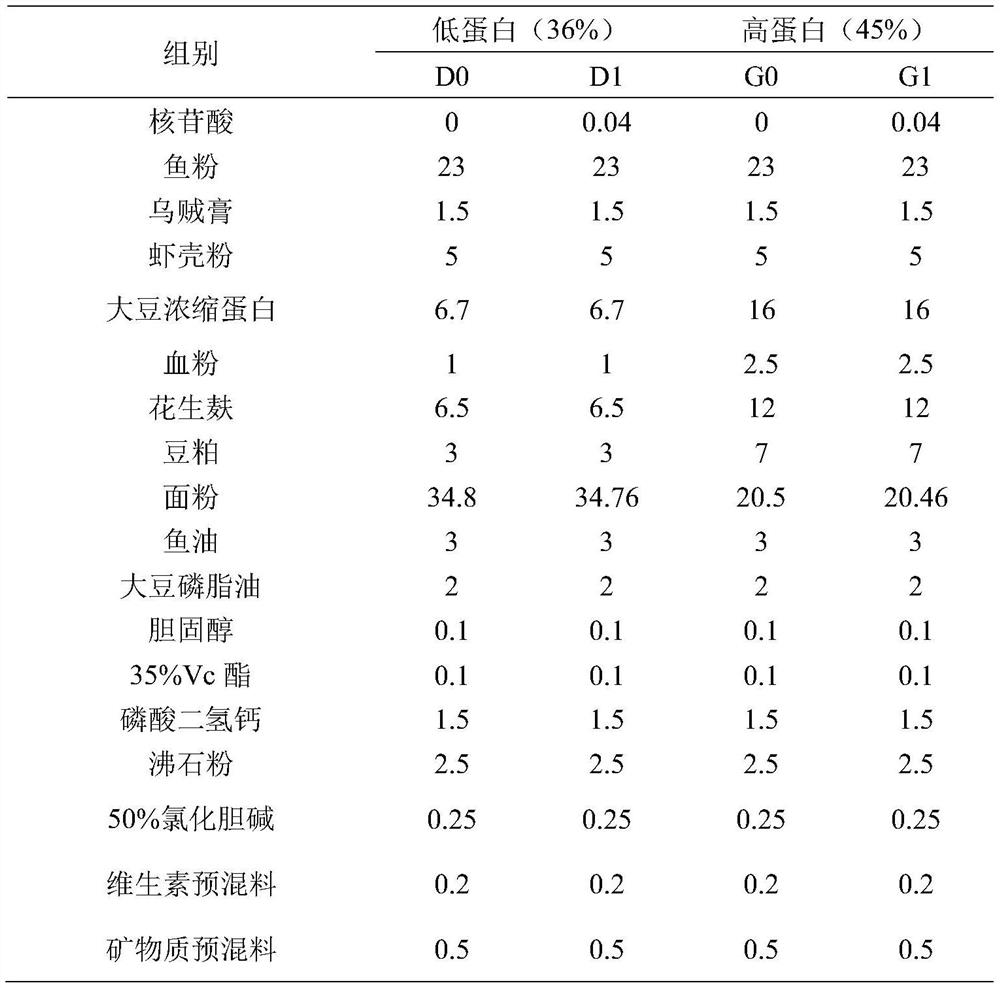
PendingCN112515054AImprove utilization efficiencySimple compositionFood processingClimate change adaptationBiotechnologyTotal protein
The invention belongs to the technical field of aquaculture, and particularly relates to a low-protein feed for improving growth performance of litopenaeus vannamei and application thereof. The low-protein feed comprise nucleotide, and is used for improving the digestion and absorption capacity of the litopenaeus vannamei to nutrient substances in feed, improving growth performance of the litopenaeus vannamei, improving the body composition and serum biochemical indexes of the litopenaeus vannamei and improving immunity of the litopenaeus vannamei. The nucleotide comprises 5'-cytidylate, 5'-adenylate, disodium 5'-inosinate, disodium 5'-uridylate, disodium 5'-guanylate and disodium 5'-thymidylate. The low-protein feed of the present invention is mainly used for improving crude fat content,serum glutamic oxalacetic transaminase and glutamic-pyruvic transaminase, hepatopancreatic glutamic-pyruvic transaminase, blood cell count, serum PO activity and the total protein content in stomachsand intestinal tracts of the litopenaeus vannamei.
Owner:ANIMAL SCI RES INST GUANGDONG ACADEMY OF AGRI SCI
Feed for treating fatty liver disease of turtles and preparation method thereof
InactiveCN105581031AEnhance immune functionReduce mortalityAnimal feeding stuffAccessory food factorsBiotechnologyManihot
The invention discloses a feed for treating a fatty liver disease of turtles and a preparation method thereof, and belongs to the technical field of turtle feed processing. The feed is prepared from, by weight, 30-50 parts of silkworm pupa meal, 50-70 parts of manihot utilissima powder, 8-16 parts of table salt, 6-14 parts of dimethyl-beta-propiothetin, 5-9 parts of betaine, 4-8 parts of glycine, 4-8 parts of cytidylic acid, 0.2-0.6 part of Tomatin polypeptide, 1-3 parts of citronellal and 15-42 parts of traditional Chinese medicine preparation. The feed is prepared through the steps of drying, smashing, screening, liquid medicine extracting, concentrating, mixing, pelletizing, sterilizing and the like. According to the feed for treating the fatty liver disease of the turtles, the effects of multiple traditional Chinese medicines are synthesized, the effects of resisting bacteria, diminishing inflammation, enhancing the body immune function and the like can be achieved, the cure rate of the fatty liver disease of the turtles can be effectively increased, and the death rate of the fatty liver disease of the turtles can be effectively decreased; the feed is reasonable in preparation, rich in nutrition and capable of increasing the feed intake of the turtles suffering from the fatty liver disease and increasing the growth speed.
Owner:张莘蔓
Nucleotide mixture powder and preparation method and application thereof
ActiveCN110468170AHigh purityImprove stabilityAccessory food factorsFermentationCytidylic AcidsChemistry
The invention discloses nucleotide mixture powder and a preparation method and application thereof. The preparation method comprises the following steps of adding a ribonucleic acid solution in nuclease liquid in a fed-batch manner, performing enzymolysis, performing concentration, and performing drying so as to obtain the nucleotide mixture powder containing uridylic acid, guanylic acid, cytidylic acid and adenylic acid. The nucleotide mixture powder provided by the invention is high in purity and good in stability, is favorable in fluidity, and can be widely applied to feed trade. The preparation method provided by the invention is high in yield, simple to operate, low in cost, and suitable for industrial production.
Owner:NANJING BIOTOGETHER
Riemerella anatipestifer bacterial ghost vaccine adopting chitosan oligosaccharide as adjuvant
InactiveCN104971346AImprove the level ofAvoid the stress of immunizationAntibacterial agentsBacterial antigen ingredientsPenicillinAdjuvant
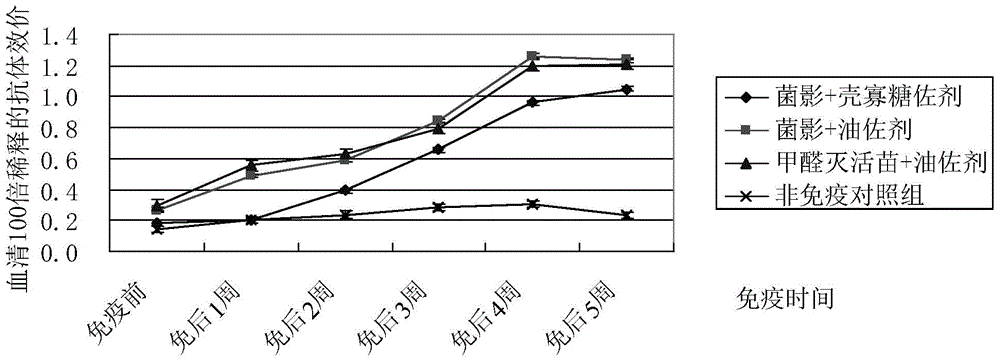
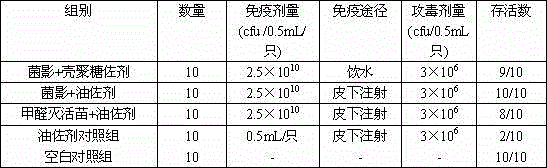
The purpose of the present invention is to provide a riemerella anatipestifer bacterial ghost vaccine loaded with polyinosinic acid-polycytidylic acid having interferon inducing activity and added with chitosan oligosaccharide as an adjuvant. According to the technical scheme, a temperature control expression vector is constructed and is electrotransformed into riemerella anatipestifer protoplast, positive clones are screened and then are subjected to bacterial amplification culture at a temperature of 28 DEG C, lysis gene expression is induced at a temperature of 42 DEG C, polylysine is added to continuously act when the OD600 is no longer be reduced so as to completely lyze the live bacteria, centrifugation is performed to separate the bacteria, the dried bacteria and a 6 mg / mL Poly I:C solution according to an equal ratio so as to make the Poly I:C be embedded into the bacterial ghosts, a 2% chitosan oligosaccharide aqueous solution is added, stirring is performed to form a uniform mixture, sub-packaging is performed into penicillin bottles, and freeze-drying is performed so as to obtain the novel riemerella anatipestifer bacterial ghost vaccine. According to the present invention, the prepared riemerella anatipestifer bacterial ghost vaccine is immunized through drinking water, such that the local immunity on the respiratory tract mucous membrane and the digestive tract mucous membrane can be irritated, the humoral immunity can be stimulated, and the protection effect on the human body is not different from the injection immunity.
Owner:QINGDAO AGRI UNIV
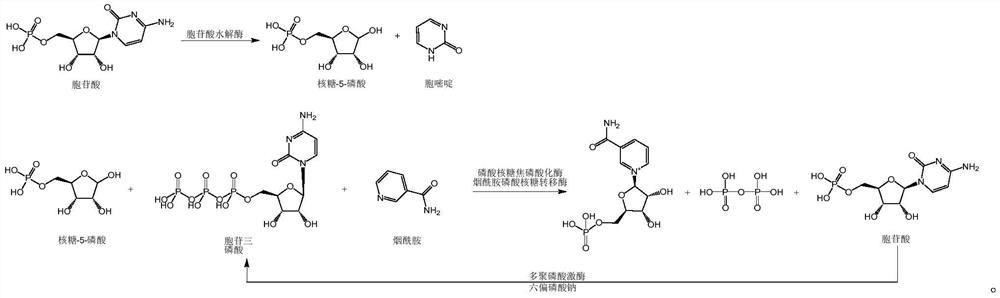
Enzymatic synthesis method of nicotinamide mononucleotide
ActiveCN112961890AGood removal effectAvoid expensiveFermentationBulk chemical productionEnzymatic synthesisNicotinamide mononucleotide
The invention discloses an enzymatic synthesis method of nicotinamide mononucleotide. The enzymatic synthesis method comprises the following steps: S1, taking ribose-5-phosphate, cytidine monophosphate, polyphosphate and nicotinamide as raw materials, and generating nicotinamide mononucleotide under the catalytic action of ribose phosphate pyrophosphorylase, polyphosphate kinase and nicotinamide ribose phosphate transferase. The applicant uses polyphosphate kinase to catalyze cytidine monophosphate to cyclically obtain cytidine triphosphate by using a phosphate group provided by the polyphosphate, and the cytidine triphosphate is used as a pyrophosphoric acid donor of the ribose phosphate pyrophosphorylase to promote the enzymatic reaction of the ribose-5 phosphate and the nicotinamide, thereby avoiding the use of expensive ATP (adenosine triphosphate). Besides, the cytidine monophosphate and the cytidine triphosphate in the enzymatic reaction process provided by the invention are almost insoluble in water under an acidic condition, so that impurities in the product can be quickly, simply and conveniently removed by directly adjusting the pH value after the reaction is finished, the purification process is simplified, and the separation and purification difficulty is reduced.
Owner:深圳希吉亚生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com