Enzymatic synthesis method of nicotinamide mononucleotide
An enzymatic synthesis, single nucleotide technology, applied in the direction of fermentation, etc., can solve the problems of high price, unfavorable enzymatic NMN industrialization, and high difficulty in separation of products and raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Enzyme preparation
[0051] According to the sequences of the following four enzyme genes of cytidylate hydrolase (phNn), phosphoribosyl pyrophosphorylase (rpPk), nicotinamide phosphoribosyltransferase (NAMPT) and polyphosphate kinase (PPK), the art A well-known design method uses primer design software to design the amplification primer pairs respectively.
[0052] Extracting Shigella sonnei (S.sonnei) genomic DNA, using it as a template, using the phNn amplification primer pair to amplify the cytidylate hydrolase fragment of Shigella sonnei, and connecting it to the pET24a vector;
[0053] Extract Pyrococcus (P.horikoshii) genomic DNA, use it as a template, utilize the rpPk amplification primer pair to amplify the phosphoribosyl pyrophosphorylase fragment of Pyrococcus, and connect it to the pET24a vector;
[0054] Extract Haemophilus ducreyi (H.ducreyi) genomic DNA, use it as a template, use the NAMPT amplification primer pair to amplify the nicotinamide phosphoribo...
Embodiment 2
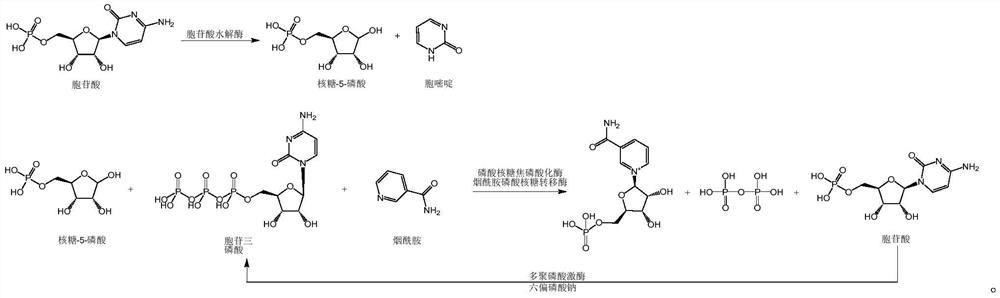
[0073] This embodiment provides an enzymatic synthesis method of nicotinamide mononucleotide, and the specific reactions that occur during the reaction process of the enzymatic synthesis method are as follows:
[0074]
[0075] The enzymatic synthesis method comprises the following steps:
[0076] S0: Add cytidylic acid with a final concentration of 100mM in 1L of pure water, adjust the pH to 7.5 with 40% sodium hydroxide solution, add 500 U of cytidylic acid hydrolase, stir at 35°C for 2 hours, and heat to 80°C , cooled and centrifuged to obtain a ribose-5-phosphate solution.
[0077] S1: Add nicotinamide and 50 mM MgCl to the ribose-5-phosphate solution obtained in S0 with a final concentration of 100 mM 2 , 60mM sodium hexametaphosphate and 4mM cytidylic acid, adjust the pH to 7.5 with 40% sodium hydroxide solution, add 1000U of phosphoribosyl pyrophosphorylase, 1000U of polyphosphate kinase and 500U of nicotinamide phosphoribosyl Transferase, stirred at 35°C for 6 hou...
Embodiment 3
[0081] This embodiment provides an enzymatic synthesis method of nicotinamide mononucleotide, comprising the following steps:
[0082] S0: Add cytidylic acid with a final concentration of 100mM in 1L of pure water, adjust the pH to 7.5 with 40% sodium hydroxide solution, add 500 U of cytidylic acid hydrolase, stir at 35°C for 2 hours, and heat to 80°C , cooled and centrifuged to obtain a ribose-5-phosphate solution.
[0083] S1: In the ribose-5-phosphate solution obtained in S0, add nicotinamide with a final concentration of 100 mM, MgCl with a final concentration of 50 mM, sodium hexametaphosphate with a final concentration of 60 mM and cytidylic acid with a final concentration of 4 mM, and use 40 % sodium hydroxide solution to adjust the pH to 7.5, add 1500 U of phosphoribosyl pyrophosphorylase, 1500 U of polyphosphate kinase and 500 U of nicotinamide phosphoribosyltransferase, and stir at 35° C. for 6 hours.
[0084] S3: After the enzymatic reaction, the pH of the solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com