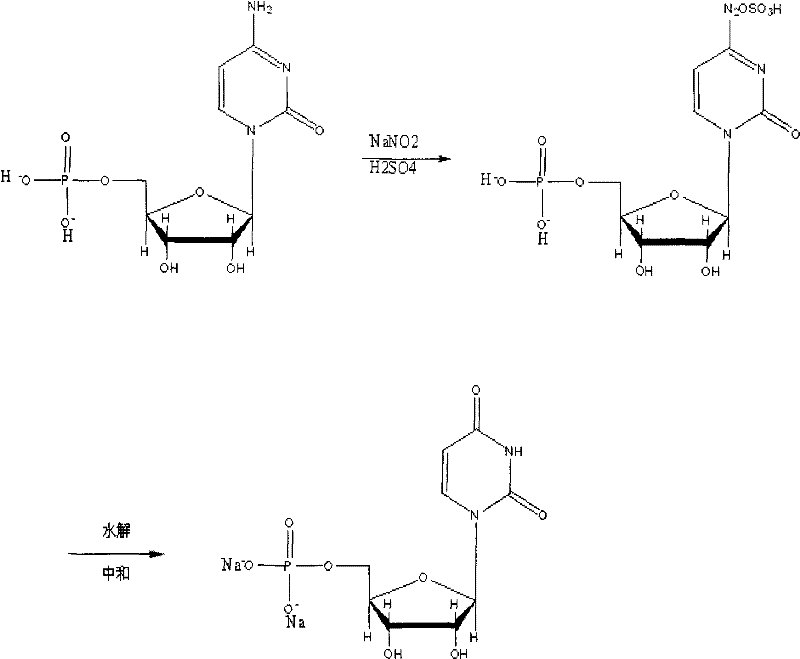
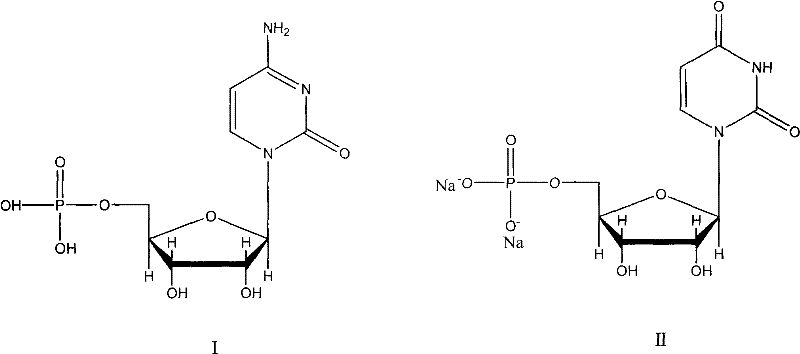
New method for synthesizing uridylic acid disodium
A technology of disodium uridine acid and a new method, which is applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of high production cost, difficult purification, low yield, etc., and achieve low production cost , stable process and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a dry 500ml four-necked bottle, install a reflux condenser and a mechanical stirring device. Add 20 grams of 5′-cytidylic acid and 100 ml of deionized water, stir to dissolve. Add 4.31 g of sodium nitrite and stir to dissolve. Use an ice-salt bath to lower the temperature of the inner liquid to -5°C, start adding sulfuric acid dropwise, and strictly control the temperature during the entire dropping process at -5°C to 0°C. A total of 40 grams of sulfuric acid is added.
[0019] After adding sulfuric acid, remove the ice-salt bath. The temperature of the above solution was slowly raised to 60° C. within 30 minutes, and after stirring for 1 hour, the reaction liquid was detected by HPLC until there was no 5′-cytidylic acid raw material, and the reaction was terminated. Subsequently, water vapor is directly introduced into the reaction flask to carry out steam distillation. Stop distillation when there are no more oil droplets in the distillate.
[0020] The collect...
Embodiment 2
[0023] In a dry 500ml four-necked bottle, install a reflux condenser and a mechanical stirring device. Add 20 grams of 5′-cytidylic acid and 300 ml of deionized water, stir to dissolve. Add 21.40 g of sodium nitrite and stir to dissolve. The temperature of the inner liquid was lowered to -5°C with an ice-salt bath, and sulfuric acid was added dropwise. During the entire dropping process, the temperature was strictly controlled at -5°C to 0°C. A total of 320 grams of sulfuric acid was added.
[0024] After adding sulfuric acid, remove the ice-salt bath. The temperature of the above solution was slowly raised to 10° C. within 30 minutes, and after stirring for 20 hours, the reaction solution was detected by HPLC to have no 5′-cytidine raw material, and the reaction was terminated. Subsequently, water vapor is directly introduced into the reaction flask to carry out steam distillation. Stop distillation when there are no more oil droplets in the distillate.
[0025] The colle...
Embodiment 3
[0028] In a dry 500ml four-necked bottle, install a reflux condenser and a mechanical stirring device. Add 20 grams of 5′-cytidylic acid and 180 ml of deionized water, stir to dissolve. Add 6.47 g of sodium nitrite and stir to dissolve. Use an ice-salt bath to lower the temperature of the inner liquid to -5°C, start adding acetic acid dropwise, and strictly control the temperature during the entire dropping process at -5°C to 0°C. Add 60 grams of acetic acid in total.
[0029] After adding acetic acid, remove the ice-salt bath. The temperature of the above solution was slowly raised to 50° C. within 30 minutes, and after stirring for 3 hours, the reaction solution was detected by HPLC to have no 5′-cytidylic acid raw material, and the reaction was terminated. Subsequently, water vapor is directly introduced into the reaction flask to carry out steam distillation. Stop distillation when there are no more oil droplets in the distillate.
[0030] The collected aqueous solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com