Composition for treating and/or preventing hepatitis b virus infection and use thereof
A composition and technology of polyamino compounds, applied in the field of treatment and/or prevention of HBV infection or diseases mediated by HBV infection, capable of solving problems such as loss of vaccine potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1. Preparation of the composition
[0074] In some embodiments, the composition comprises HBsAg, PIC, kanamycin, and calcium chloride, wherein the ratio of HBsAg to PIC is 1:25. More specifically, the composition comprises 40 μg / unit dose of HBsAg, 1000 μg / unit dose of PIC, 800 IU / unit dose of kanamycin, and 0.16 μmol / unit dose of Ca2 + .
[0075] In some embodiments, the composition comprises HBcAg, HBsAg, PIC, kanamycin, and calcium chloride. The composition comprises 40 μg / unit dose of HBsAg, 1000 μg / unit dose of PIC, 800 IU / unit dose of kanamycin and 0.16 μmol / unit dose of Ca2 + . More specifically, the concentration of HBcAg ranges from 10 μg / unit dose to 50 μg / unit dose.
[0076] In some embodiments, the composition comprises HBcAg, HBsAg, PIC, kanamycin, and calcium chloride. The composition comprises 40 μg / unit dose of HBsAg, 1000 μg / unit dose of PIC, 800 IU / unit dose of kanamycin and 0.16 μmol / unit dose of Ca2 + . More specifically, the concen...
Embodiment 2
[0077] Example 2. Preclinical Toxicology Studies
[0078]Example 2 describes the preclinical toxicity of compositions according to some embodiments of the present disclosure and their major components, including PIC, at least one antibiotic or polyamino compound, and at least one positive ion, collectively referred to as "PIKA". Science research. As shown in Table 1, the mice used in Example 2 had an average body weight of 0.019 kg and were able to tolerate 0.2 ml of a composition comprising 8 μg of HBsAg (0.4 mg / kg) and 200 μg of PIC (10 mg / kg); Or able to tolerate 0.2 ml PIKA containing 200 μg PIC (10 mg / kg). The dose of the composition used in mice was approximately 1,200 times higher than the dose recommended for use in humans (20 μg HBsAg (0.0003 mg / kg) and 500 μg PIKA (0.0083 mg / kg)). The highest recommended human dose is twice the lowest recommended human dose, which would still provide a margin of safety (MoS) of 600. Based on chronic toxicology studies in rodent ra...
Embodiment 3
[0081] Example 3. Preclinical Study of Rodent Immune Response
[0082] experiment method:
[0083] The rodents used in Example 3 were 6-week-old BALB / c mice, which were divided into 6 groups. Each group included 18 mice (9 males, body weight range 17.3 g to 20.7 g; 9 females, body weight range 19.0 g to 23.7 g). As shown in Table 2, the compositions and other substances were administered by tibialis anterior muscle injection on days 1, 8, 22, 36, 50, 64 and 78.
[0084] Table 2. Administration regimen
[0085]
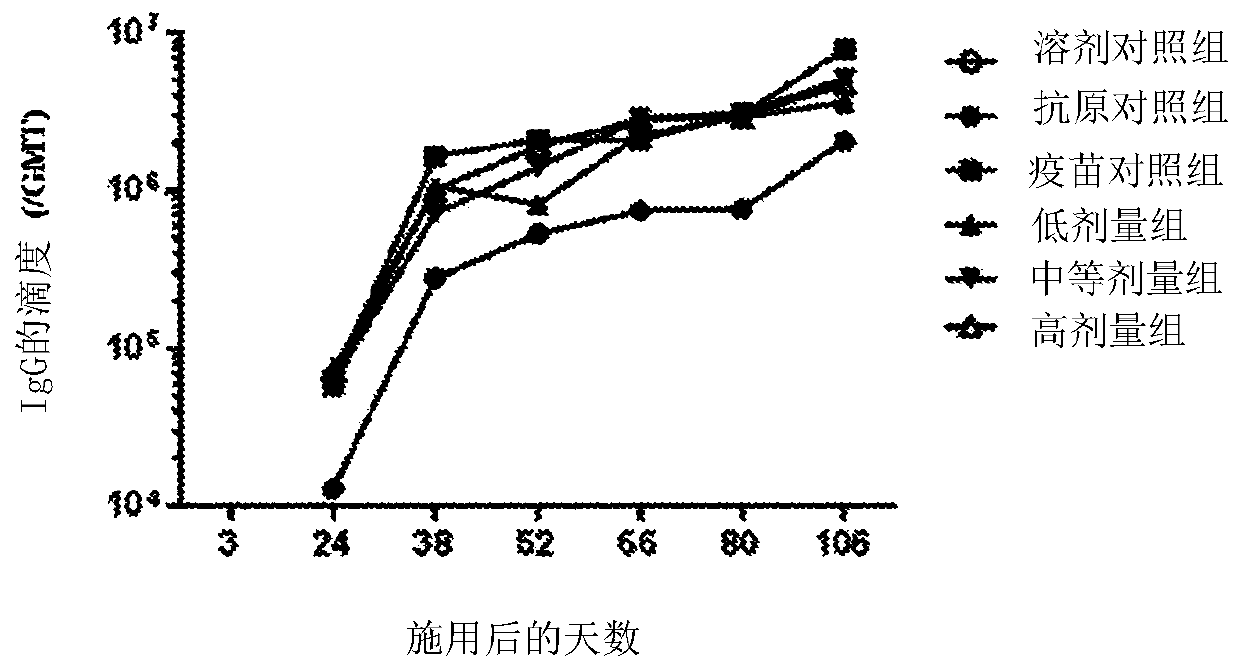
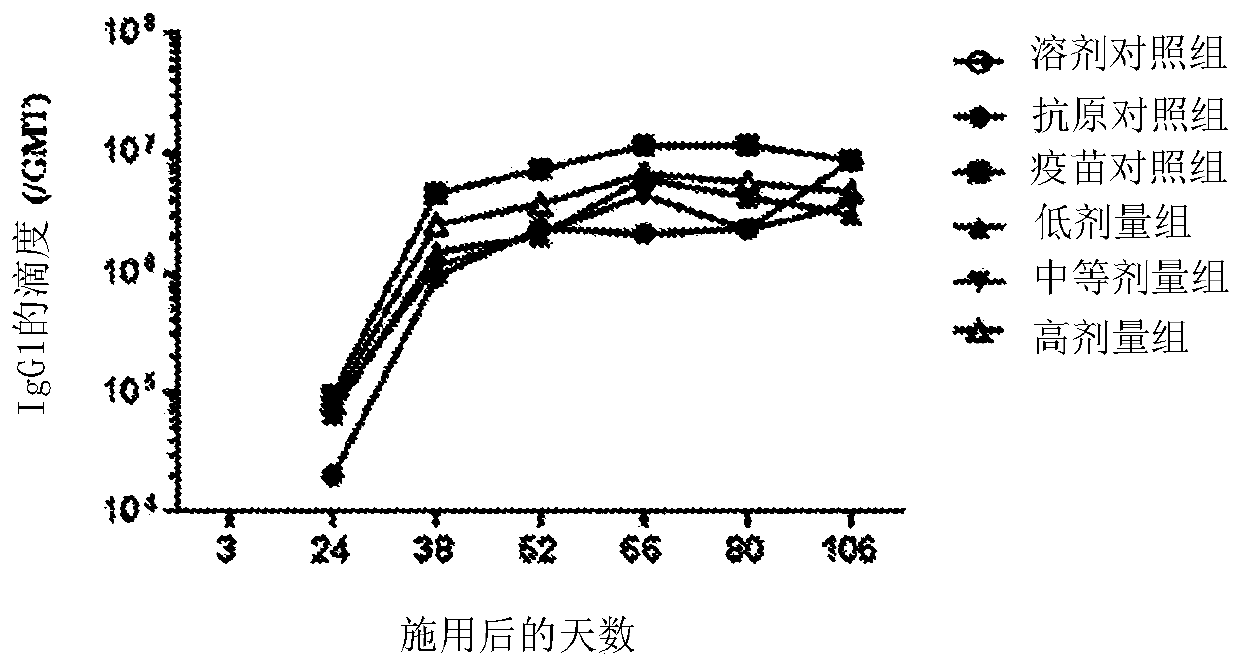
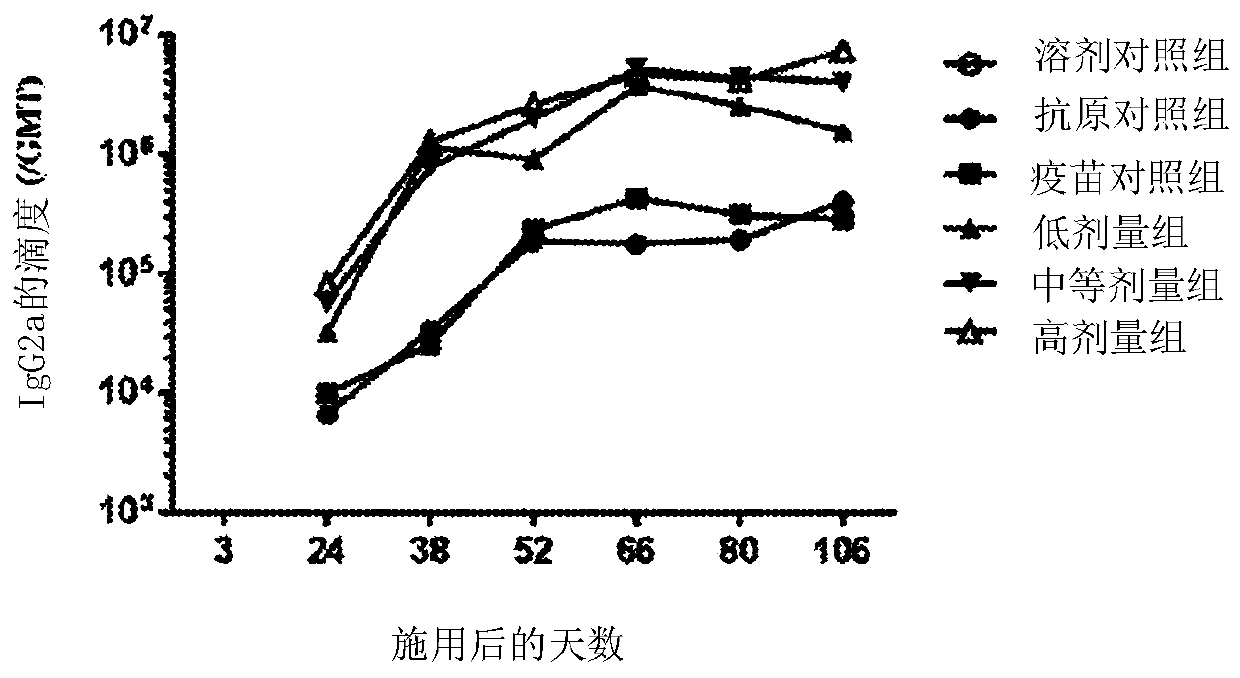
[0086] On days 3, 24, 38, 52, 66, 80, and 106, blood samples were collected and titers of anti-HBsAg antibodies were measured to determine the humoral immune response.
[0087] On days 38, 80 and 106, 3 male and 3 female mice from each group were sacrificed to determine T cell mediated immune responses. with HBsAg or HBsAg CD8 + Peptides stimulate splenocytes ex vivo. The frequency of IFN-γ producing splenocytes was measured by ELISPOT.
[0088] result:
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com