Synthetic method of citicoline sodium
A technology of citicoline sodium and its synthesis method, which is applied in the field of synthesis of citicoline sodium, and can solve problems such as high synthesis cost and limited enzyme source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
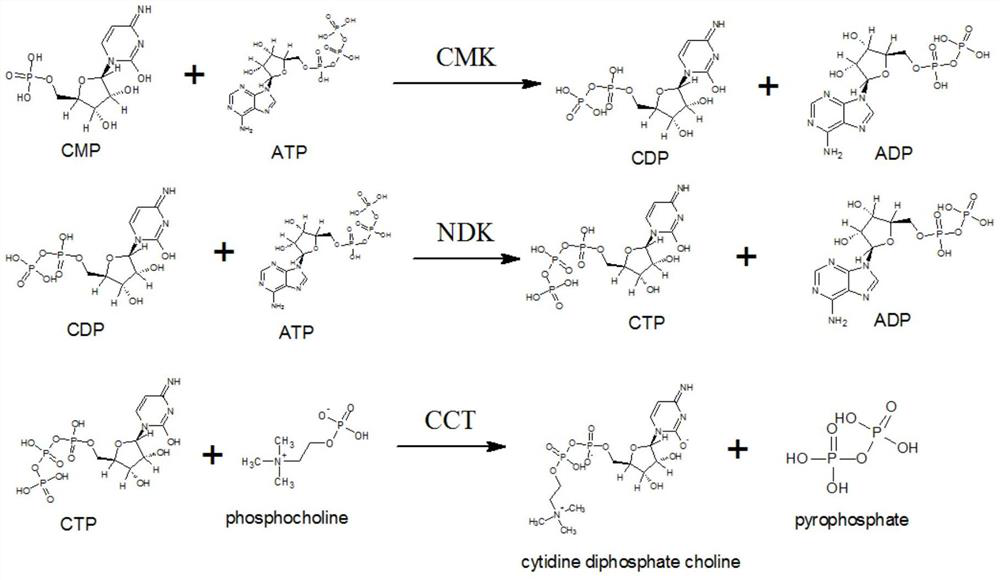
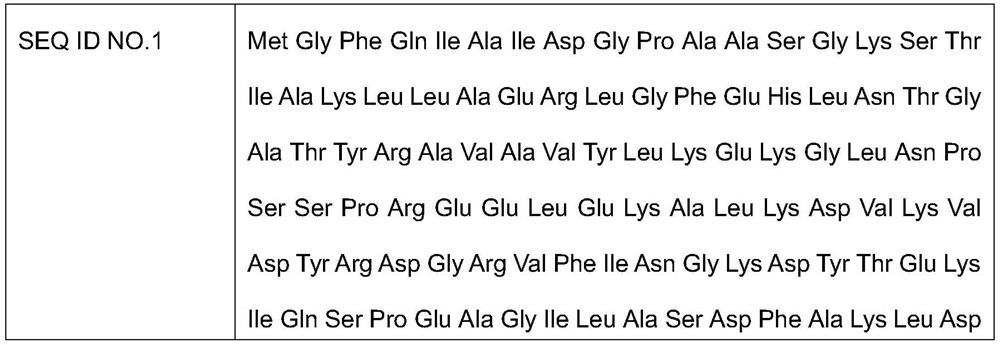
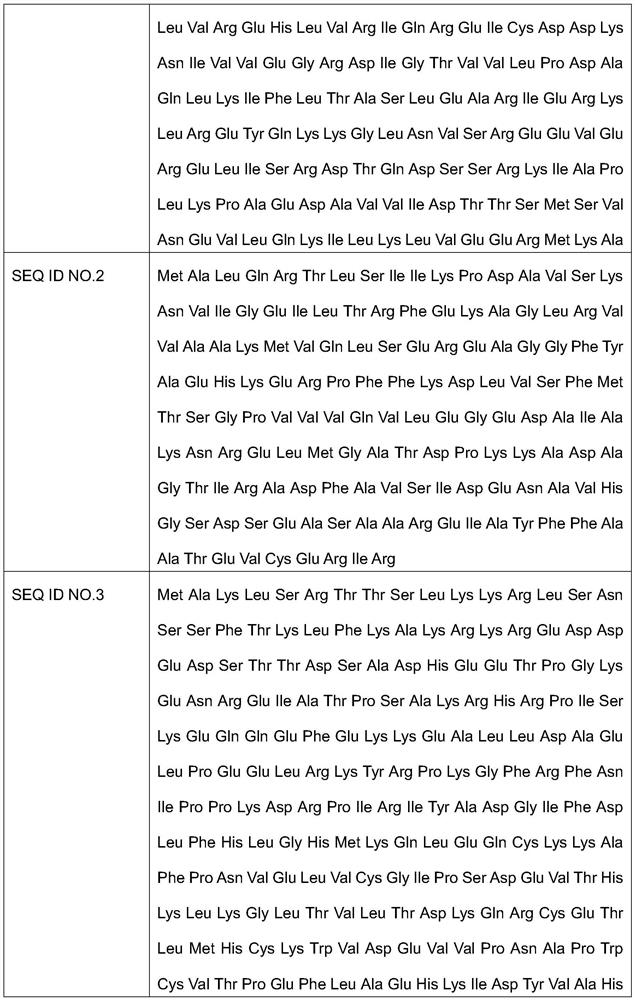
[0036] According to the synthetic method of citicoline sodium described in the preferred embodiment, it mainly comprises the following steps: utilize the Escherichia coli engineering bacteria of molecular cloning CMP phosphokinase, nucleoside diphosphate kinase, phosphorylcholine-cytidylyl transferase and prepare The above 3 kinds of enzymes, and provide the supernatant of the enzyme liquid of the 3 kinds of enzymes; using phosphorylcholine chloride calcium salt, ATP, and CMP as raw materials, the above three kinds of enzymes are jointly catalyzed to generate citicoline sodium, and the used The amino acid sequence of the CMP phosphokinase shown in SEQ ID NO.1, the amino acid sequence of the nucleoside diphosphate kinase used is shown in SEQ ID NO.2, the amino acid sequence of the phosphorylcholine-cytidylyl transferase used The sequence is shown in SEQ ID NO.3.
[0037] In a preferred embodiment, the synthetic method of described citicoline sodium comprises the following steps...
Embodiment
[0051] Prepare citicoline sodium according to the following specific steps successively:
[0052] I construct 3 types of strains respectively :
[0053] Three genes (genes of these three types of enzymes) that are active against specific substrates were found through gene search, and the corresponding base sequences were synthesized by a gene company and cloned into the pACYC-Duet-1 vector. We transformed the received plasmid into Escherichia coli BL21 (DE3) (laboratory preservation host), and in chloramphenicol (Cam + )-LB plates were screened to get positive colonies.
[0054] Use a single colony as a template to perform PCR amplification with primer 1 and primer 2. The amplification conditions include: initial denaturation at 95°C for 5 minutes, and then perform the following reaction conditions: 95°C for 30s, 55°C for 30s, 72°C for 90s, 30 cycles , and finally extended at 72°C for 3 minutes to obtain a PCR product of the expected size; the sequencing of the PCR product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com