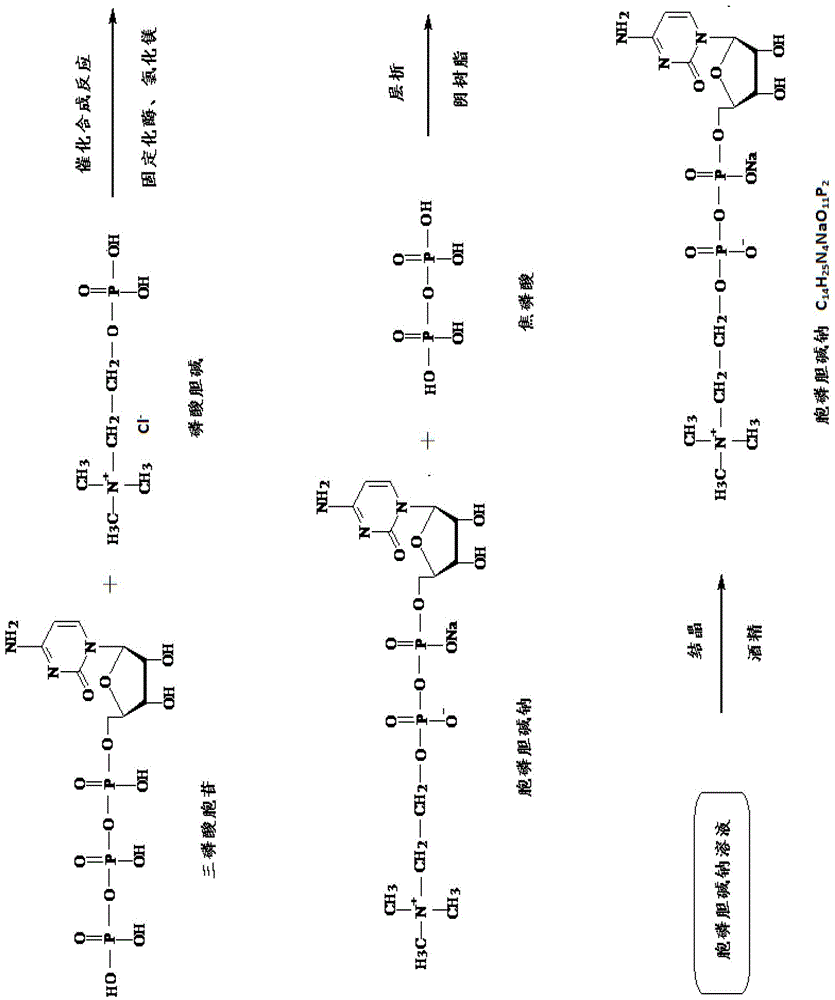
A kind of method that immobilized enzyme catalyzes to produce citicoline sodium
A technology of citicoline sodium and immobilized enzyme, which is applied in the field of immobilized enzyme catalyzed production of citicoline sodium, which can solve the problems of enzymatic synthesis, low reaction conversion rate, and low product concentration, etc., and achieve reduction Effects of synthesis process time, environmental friendliness, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for producing citicoline sodium catalyzed by immobilized enzymes, comprising the steps of:
[0042] (1) Engineering bacteria fermentation:
[0043] Inoculate 150ml seed liquid of Escherichia coli engineering bacteria with molecularly cloned cytidine phosphotransferase gene into a 3L culture medium system for 26 hours. The culture medium system contains peptone 10g / L, yeast extract 10g / L, glucose 5g / L L, inorganic salt 8g / L, supplemented with 150g glucose, 15g peptone and 15g yeast extract during the dissolution period in purified water; after the fermentation was completed, the fermentation broth was centrifuged to collect 152g of bacterial cells.
[0044] (2) Preparation of cytidine phosphotransferase serum:
[0045] Take 30g of bacteria, mix and suspend it with 180ml phosphate buffer solution, and use ultrasonic crushing for 30 minutes to obtain a broken liquid. Add ammonium sulfate to the broken liquid to 30% saturation for salting out, and then use 200nm mic...
Embodiment 2
[0053] A method for producing citicoline sodium catalyzed by immobilized enzymes, comprising the steps of:
[0054] (1) Engineering bacteria fermentation:
[0055] Inoculate 200ml of Escherichia coli engineered bacteria seed solution with molecularly cloned cytidine phosphotransferase gene into a 3L medium system (the medium system contains 15g / L peptone, 15g / L yeast extract, 8g / L glucose, and 13.5g inorganic salt / L, dissolved in purified water) for 28 hours, supplemented with 288g glucose, 30g peptone and 30g yeast extract during this period. After the fermentation was completed, 165 g of bacterial cells were collected by centrifugation of the fermented liquid.
[0056] (2) Preparation of cytidine phosphotransferase serum:
[0057] Take 20g of bacteria, mix and suspend it with 200ml phosphate buffer solution, and then crush it with ultrasonic wave for 30 minutes to obtain a crushed liquid. Add ammonium sulfate to the crushed liquid to 45% saturation, and then use 500nm mic...
Embodiment 3
[0065] The preparation of citicoline sodium comprises the following steps:
[0066] (1) Engineering bacteria fermentation:
[0067] Inoculate 300ml of Escherichia coli engineered bacteria seed solution with molecularly cloned cytidine phosphotransferase gene into a 3L medium system (the medium system contains 20g / L of peptone, 20g / L of yeast extract, 8g / L of glucose, 15g / L of inorganic salt L, the balance is dissolved in purified water) for 32 hours, during which 360g glucose, 48g peptone and 48g yeast extract were supplemented. After the fermentation was completed, 187 g of bacterial cells were collected by centrifugation of the fermented liquid.
[0068] (2) Preparation of cytidine phosphotransferase serum:
[0069] Take 30g of bacterial cells, mix and suspend them with 120ml phosphate buffer solution, crush the bacterial solution by ultrasonic wave for 30 minutes to obtain a broken liquid, add ammonium sulfate to the broken liquid to a saturation of 25%, and then use 500n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com