Immunogenic substances comprising a polyinosinic acid-polycytidilic acid based adjuvant
a polynucleotide and immunogenic composition technology, applied in the field of immunogenic compositions, can solve the problems of ineffective use of polyinosinic acid-polycytidylic acid (pic), one of the most studied polynucleotide complexes, and inability to provide optimal efficacy/safety profile,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
PIKA in Combination with a Variety of Antigens Induces a Specific Immune Response
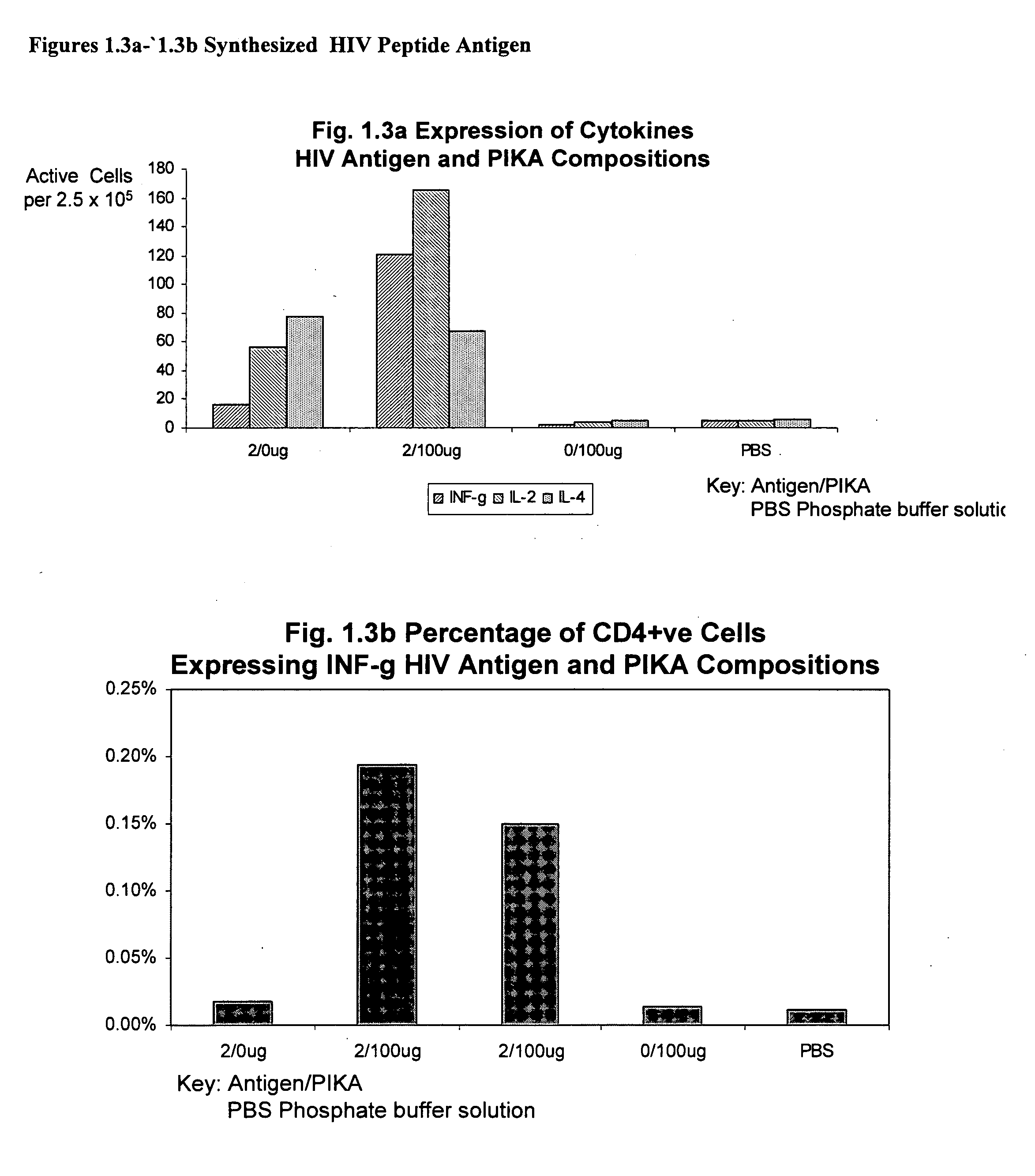
[0215] This example involves use of PIKA in combination with a variety of antigens to elicit a specific immune response in vivo. The research was conducted in a series of independent experiments with a common protocol though using a different antigen each time. The antigens tested include: a recombinant protein hepatitis B surface antigen type adw, an inactivated split influenza vaccine (VAXIGRIP from Sanofi Pasteur), a synthesized HIV peptide antigen, a recombinant protein herpes simplex virus type 2 gD antigen, recombinant protective anthrax protein antigen, inactivated whole virus avian influenza antigen strain H5N1 and an inactivated whole virus Severe Acute Respiratory Syndrome (SARS) inactivated antigen.
[0216] The protocol for the individual experiment involve the inoculation of Balb / c mice with compositions of antigen alone, antigen with the PIKA adjuvant (a heterogeneous composition of PIKA mo...
example 1.1
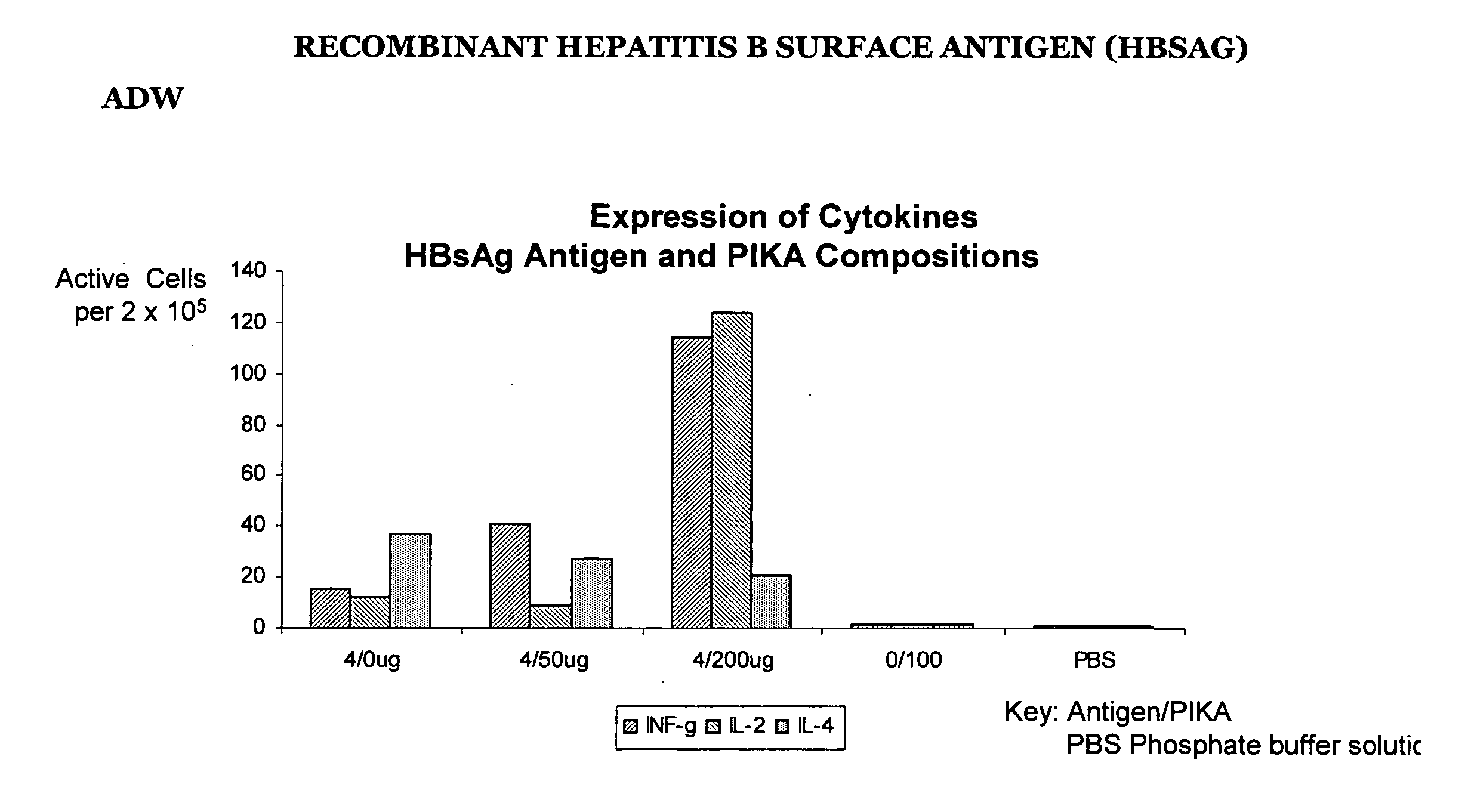
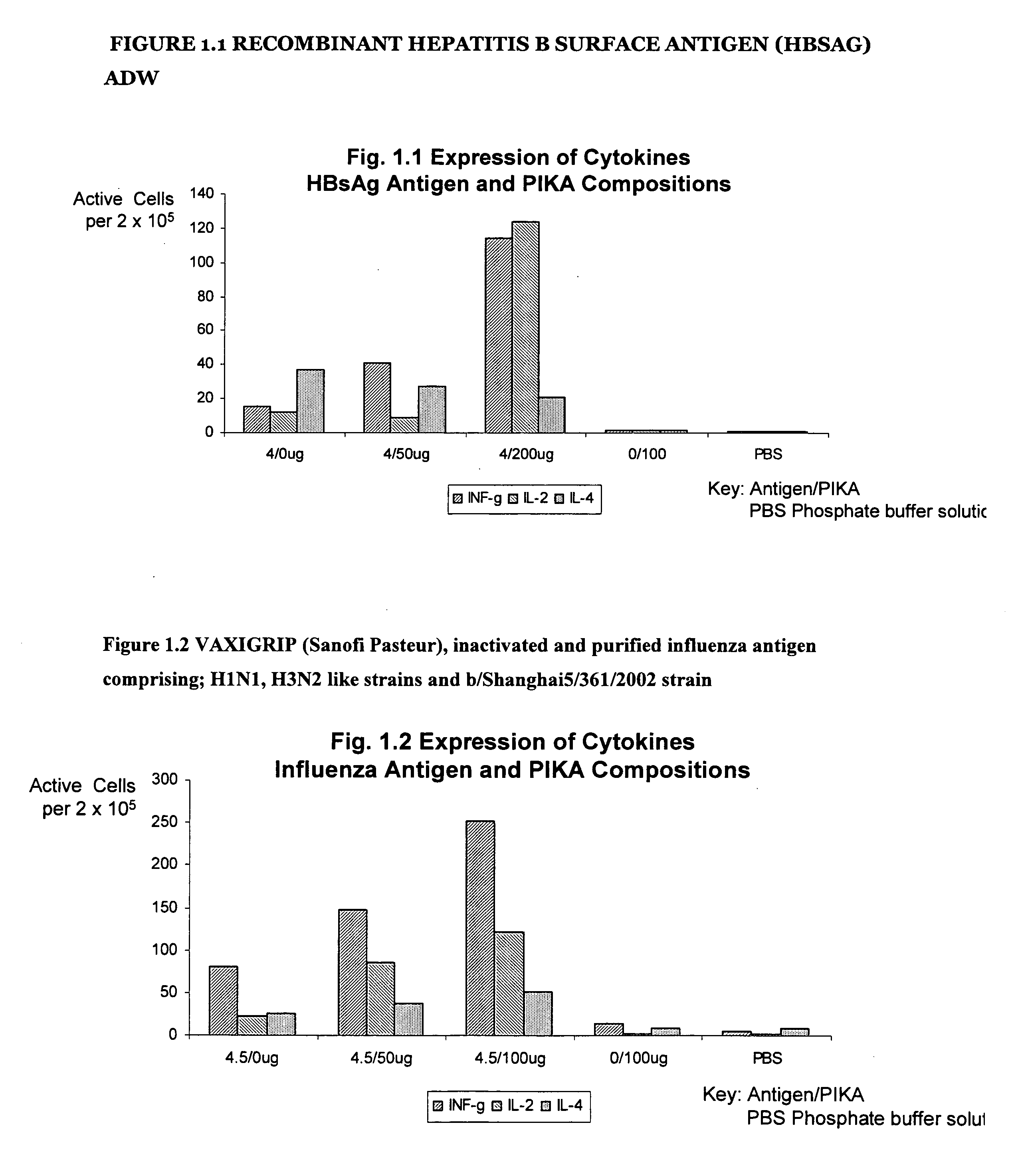
Recombinant Hepatitis B Surface Antigen (HBSAg) adw
[0222] The results in table II below are the results of the ELISPOT test detecting the presence number of cells producing INF-γ, IL-2 and IL-4 using a recombinant protein hepatitis B surface antigen (HBsAg) type adw. Groups A to E represent the different combinations of antigen / adjuvant / control media administered to the mice. The numbers in the table II (see also FIG. 1.1) represent the ELISPOT reading, the number of spot forming cells, that is, a direct measure the number of cells producing cytokine.
[0223] The distinct increase in the number of spot forming cells with the addition of the PIKA adjuvant (as compared with the antigen alone) demonstrates that the addition of the PIKA adjuvant to recombinant hepatitis B surface antigen enhances the expression of cytokines INF-γ, IL-2 and IL-4 by cultured spleen cells. The observed expression of cytokines indicates an enhanced adaptive immune response of both a humoral and cell mediate...
example 1.2
VAXIGRIP (Sanofi Pasteur), Inactivated and Purified Influenza Antigen Comprising; H1N1, H3N2 Like Strains and b / Shanghai5 / 361 / 2002 Strain
[0225] The results in table III below are the results of the ELISPOT test detecting the presence the number of cells producing INF-γ, IL-2 and IL-4 using VAXIGRIP vaccine a inactivated split human influenza vaccine produced by Sanofi Pasteur. Groups A to D represent the different combinations of antigen / adjuvant / control media administered to the mice. The numbers in the table III (see also FIG. 1.2) represent the ELISPOT reading, the number of spot forming cells, that is, a direct measure of cytokine production.
[0226] The distinct increase in the number of spot forming cells with the addition of the PIKA adjuvant (as compared with the antigen alone) demonstrates that the addition of the PIKA adjuvant to the influenza antigen enhances the expression of cytokines INF-γ, IL-2 and IL-4 by cultured spleen cells. The observed expression of cytokines in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com