Subunit vaccine against Mycobacterium tuberculosis based on modification by arabinogalactan-polyinosinic acid polycytidylic acid and preparation method thereof
A technology of polyinosinic acid cytidylic acid and arabinogalactan, which is applied in the field of Mycobacterium tuberculosis subunit vaccines, can solve problems such as toxic and side effects, and achieve the effects of preventing tuberculosis, enhancing immune response, and prolonging circulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Preparation of CT fusion protein
[0024] (1) Construction of Ag85B-HspX recombinant plasmid
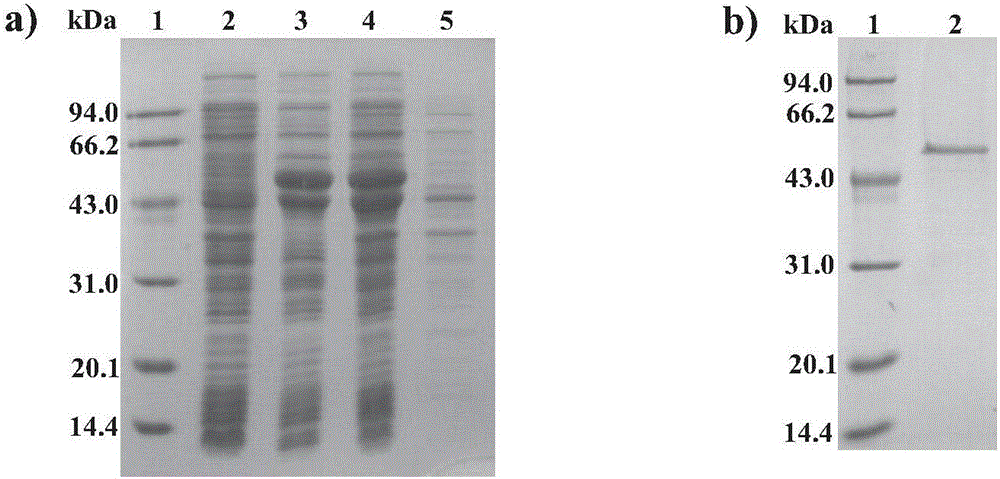
[0025] First, according to the Ag85B coding gene sequence of the standard Mycobacterium tuberculosis strain Rv1886c and the HspX coding gene sequence of Rv2031c in GenBank, three Gly-Gly-Gly-Gly-Gly-Ser (Gly-Gly-Gly-Ser) repeats were designed in the middle The whole gene sequence of the unit was then synthesized; then primers were designed, the whole gene sequence was amplified by PCR, the target gene was recovered with a gel recovery kit, and the whole gene sequence was double-digested with HindⅢ and MscⅠ. At the same time, the carrier plasmid pET26b was double-enzymatically digested, and after the target gene fragments were recovered separately, they were ligated with T4 ligase; finally, the ligated products were transformed into E. Coli BL21 (DE3), positive clones were screened by colony PCR and sequenced for identification, thereby The pET26b-Ag85B-HspX reco...
Embodiment 2
[0034] Embodiment 2: Preparation of Mycobacterium tuberculosis subunit vaccine
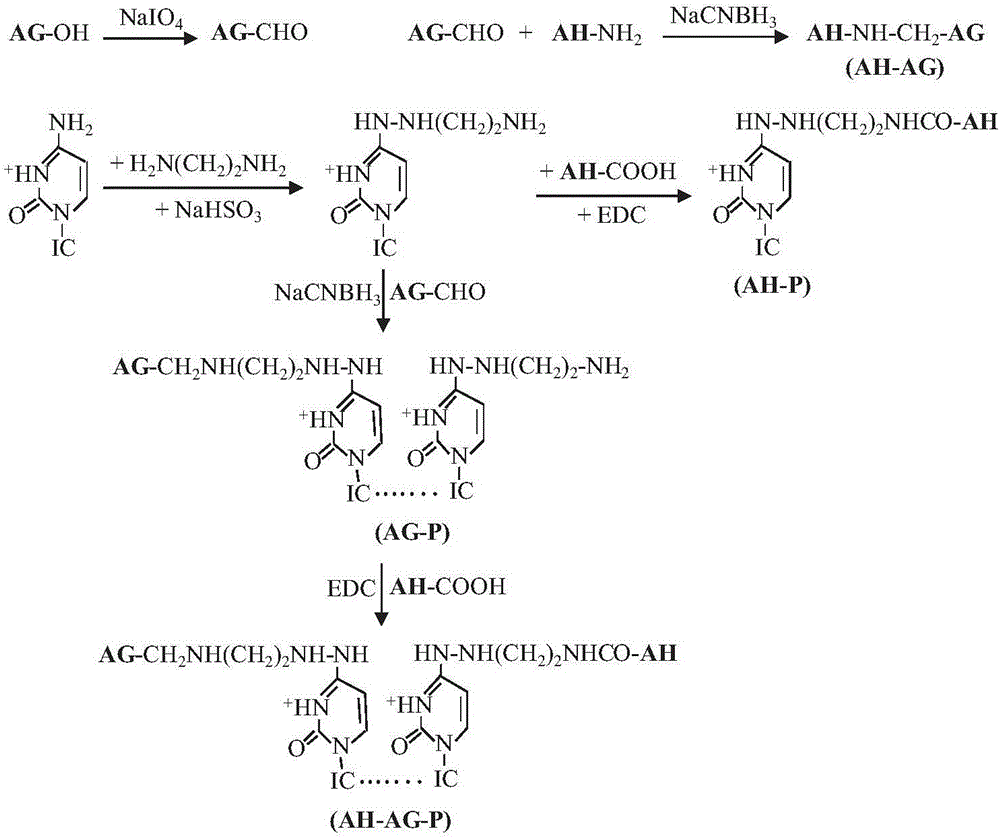
[0035] (1) Derivation of poly(I:C)
[0036] Weigh 9.98g of ethylenediamine and 2.60g of sodium bisulfite, dissolve in 25ml of deionized water, mix well, then adjust the pH of the solution to 5.5-6.0 with sodium hydroxide, add 12.5mg of poly(I:C), well mixed. Then react at 42°C for 3 hours ( figure 2 ). After the reaction, the reaction solution was fully dialyzed in deionized water with a dialysis bag with a molecular weight cut-off of 8-12 kDa, and then freeze-dried for future use.
[0037] (2) Preparation of AG-P conjugates
[0038] Weigh 10 mg AG and dissolve in 20 mM acetic acid-sodium acetate buffer solution (pH 5.5-6.0). Sodium periodate was added to make the final concentration 20 mM, and after reaction at room temperature in the dark for 20 minutes, a small amount of ethylene glycol was added to terminate the reaction. Subsequently, it was dialyzed three times for 12 hours in 20 mM p...
Embodiment 3
[0045] Example 3: Structural Characterization of Vaccines
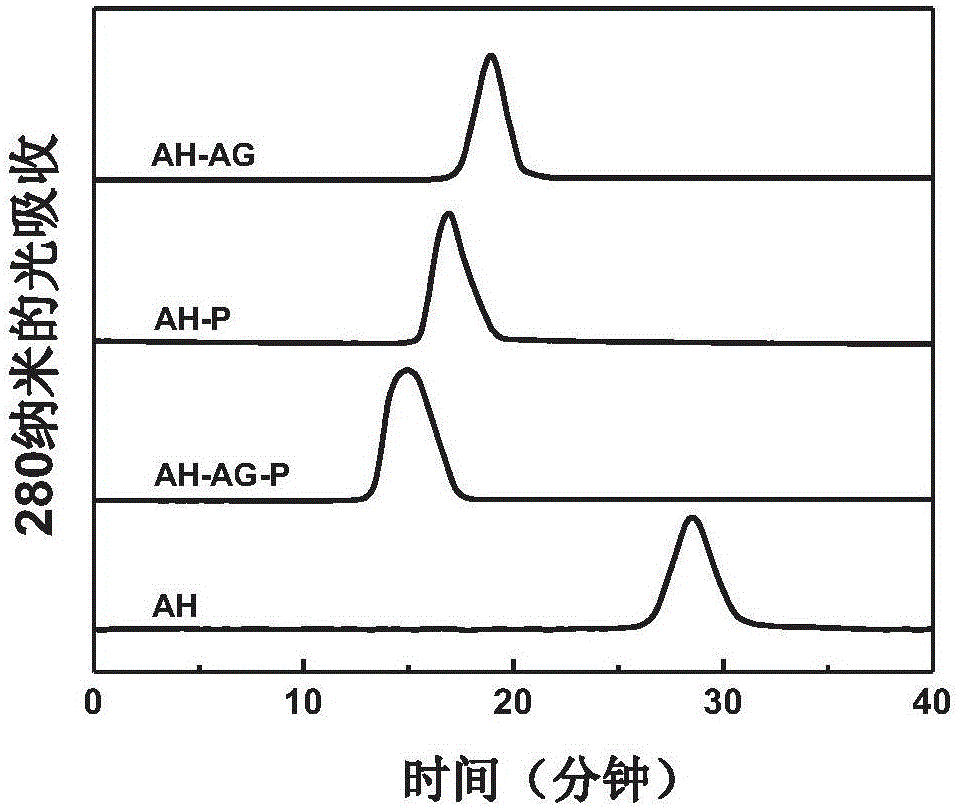
[0046] The three vaccines were identified by analytical Superdex 200 gel filtration column (1.0×30cm), the eluent was 20mM phosphate buffer (pH 7.4) containing 0.15M sodium chloride, and the flow rate was 0.5ml / min. Such as image 3 As shown, compared with the fusion protein AH, the peak times of AH-AG, AH-P and AH-AG-P were all significantly earlier. Among them, the peak time of AH-AG-P was earlier than that of AH-AG and AH-P, indicating that the modification of AH by AG-P conjugates significantly increased the molecular weight of AH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com