Novel nucleic acid adjuvant system for subunit vaccine and application of novel nucleic acid adjuvant system
A technology of subunit vaccine and nucleic acid, applied in the field of vaccine adjuvant system and subunit vaccine new nucleic acid adjuvant system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] BALB / c mice (6-8 weeks old, 14-20 g, purchased from the Laboratory Animal Center, Institute of Medical Biology, Chinese Academy of Medical Sciences) were used in this experiment, and the content of each component used in the mouse immunization test was the human dose 1 / 10 of . Immune antigen protein and adjuvant system (per injection), the specific content of each component is shown in Table 1.
[0035] Table 1
[0036]
[0037] Materials: Immune antigen protein, S1 protein was purchased from Beijing Yiqiao Shenzhou (cat: 40591- V08H); low molecular weight Poly I:C double-stranded polycytosine nucleotide fragment was purchased from (Inviv oGen, Inc. San Diego, CA , USA); GC-rich single-stranded oligodeoxynucleotide fragments (CpG ODN) include 2 nucleic acid sequences:
[0038] 2395: 5'-tcgtcgttttcggcgcgcgcc-3'; (SEQ ID NO. 1);
[0039] BW006: 5'-tcgacgttcgtcgttcgtcgttc-3'; (SEQ ID NO. 2).
[0040] Use after mixing 2395 and BW006 with the same quality;
[0041] C...
Embodiment 2
[0042] Example 2 Immunization schedule of different components
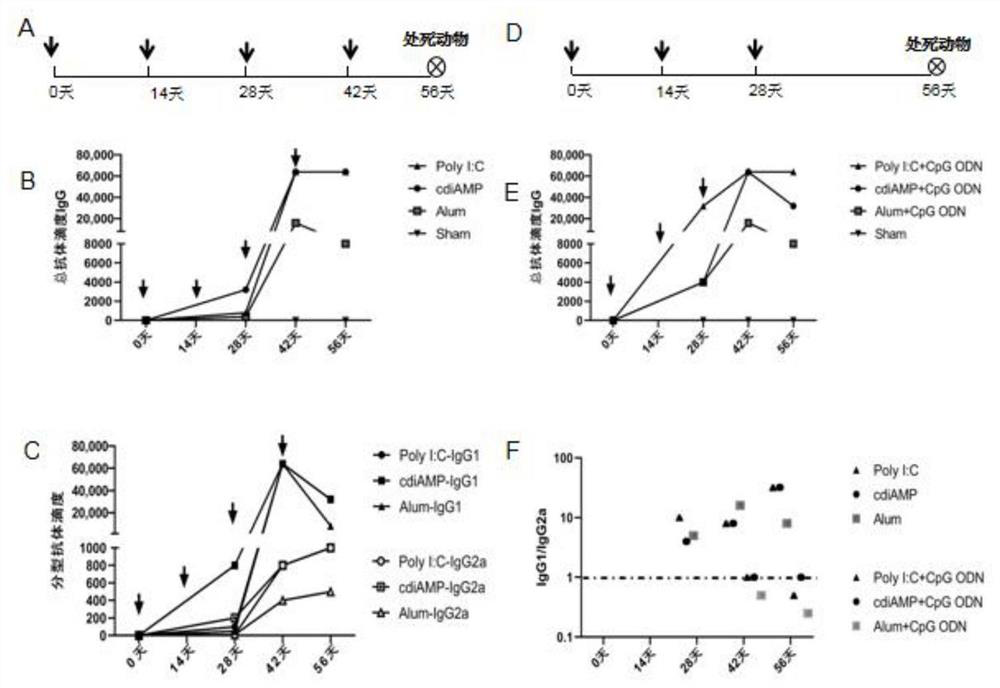
[0043] Single-component immunization program 1: The immunization components used the serial numbers 1-4 in Table 1 above, and the intramuscular injection was performed for 3 times, with an interval of 2 weeks for each injection, and the mice were sacrificed at an interval of 2 weeks after the third injection of immunization. immunization schedule see figure 1 -A.
[0044] Mixed component immunization program 2: The immunization component was used in groups No. 5-7 in Table 1 above, and 3 injections were administered intramuscularly, with an interval of 2 weeks between each injection, and the mice were sacrificed at an interval of 2 weeks after the third immunization. immunization schedule see figure 1 -A.
[0045] Mixed component immunization program 3: The immunization components were used in groups 5-7 in Table 1 above, intramuscular injection was performed for 3 times, and the interval between each injectio...
Embodiment 3
[0047] Embodiment 3 Animal experiment and detection sampling
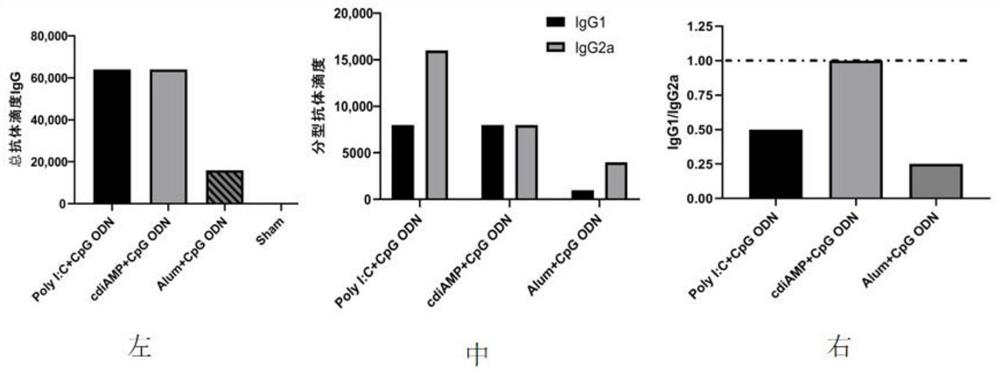
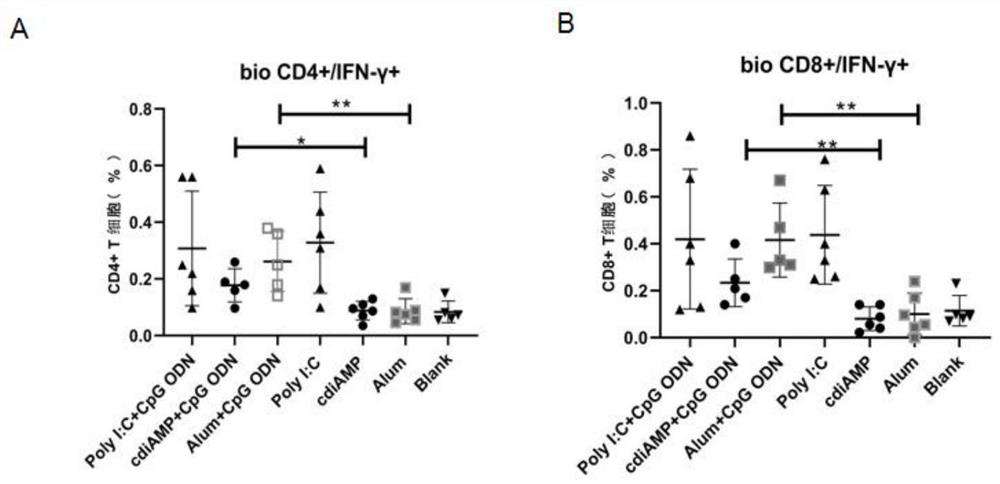
[0048]Each group was immunized with BALB / c mice (6-8 weeks old, 14-20 g, purchased from the Experimental Animal Center, Institute of Medical Biology, Chinese Academy of Medical Sciences) by intramuscular injection according to Example 2. Blood was obtained by cardiac puncture after the immunization expired or from the second needle after immunization, and blood was collected from the tail vein before each immunization. Then placed at 4°C overnight, centrifuged at 3000rpm for 20 minutes to separate the immune serum, and used ELISA to detect the total antibody titer IgG. The test results are shown in figure 1 left, figure 2 left, Figure 4 -B. Figure 4 -E, Typing antibody titer IgG1, IgG2a test results figure 1 middle, figure 2 middle, Figure 4 - C and the ratio of immune responses biased towards IgG1 / 2a figure 1 right, figure 2 right, Figure 4 -F. At the same time, the spleen lymphocytes were separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com