Largemouth bass iridovirus disease inactivated vaccine and preparation method thereof
A technology of inactivated vaccines and iridescent viruses, applied in biochemical equipment and methods, vaccines, viruses, etc., can solve the problems of largemouth bass economic losses, threats to the healthy development of the industry, and high lethality, and achieve good immune protection effects and production Low cost and good safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The present embodiment provides a kind of preparation method of largemouth bass iridovirus disease inactivated vaccine, comprises the following steps:
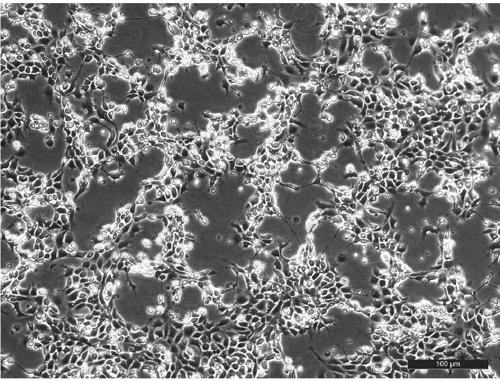
[0027]Step 1, EPC cell culture: Take 1 bottle of EPC that is full of monolayers, discard the old medium in a sterile ultra-clean bench, add 2ml of trypsin digestion solution with a concentration of 0.25% (V / V) to each bottle, and digest for 1min Quickly add 2ml of 199 medium containing 10% (V / V) calf serum with a pH of 7.4, gently blow the bottom of the cell bottle with a pipette, and then add 6ml of 199 medium containing 10% (V / V) calf serum medium to suspend cells. Take 2 T25 cell culture flasks, add 4ml of cell suspension to each flask, and culture them in a 25°C incubator. After the cells form a confluent monolayer and cover the culture dish, EPC cells are obtained for virus amplification.
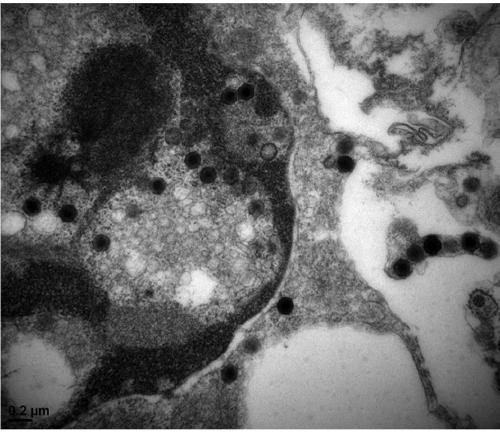
[0028] Step 2, LMBV virus amplification: suck out the culture medium in the EPC cells, and then add the infected virus suspen...
Embodiment 2
[0034] Determination of LMBV inactivation conditions and safety test.
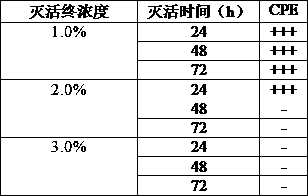
[0035] Obtain the amplified virus stock solution according to the method of Example 1, divide it into 3 equal parts, add BEI to the final concentration of BEI respectively 1%, 2%, and 2%, and inactivate it in a constant temperature shaker at 37°C at 120r / min for 24h, 48h After 72 hours, the inactivation was terminated with the same concentration of sodium thiosulfate solution and samples were taken. Take the prepared vaccine and inoculate EPC cells according to the above virus propagation method, and set up a negative control at the same time, observe for 7 to 10 days, if cytopathic effect appears, it indicates that the virus inactivation is not complete; if no cytopathic effect is seen, then blindly pass 2 Second, if cytopathy occurs in the blind transmission, it indicates that the virus inactivation is still incomplete. If no cytopathy occurs in the two blind transmissions, it indicates that the virus in...
Embodiment 3
[0041] Safety Test of Inactivated Vaccines
[0042] Sterility test: Take the vaccine prepared in Example 1, inoculate the brain infusion bacterial culture medium (BHI) plate, smear the plate with the streak method and incubate at 30°C for 15 days. If there are colonies growing, it indicates that the vaccine has bacterial contamination ; if no colonies grow, the vaccine is sterile.
[0043] Fish body safety test: Take the vaccine prepared above and inject 30 healthy largemouth bass of 50g to 70g, the injection dose is 0.1 to 0.2ml / tail, and the negative control is injected with the same dose of normal saline. After feeding for 15 to 30 days, if the vaccine group died or had clinical symptoms, but the negative control group did not show clinical symptoms or died, it indicated that the vaccine was unsafe; if neither the vaccine group nor the negative control group showed clinical symptoms or died, it indicated that the vaccine Safety.
[0044] Stress test and feeding effect aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com