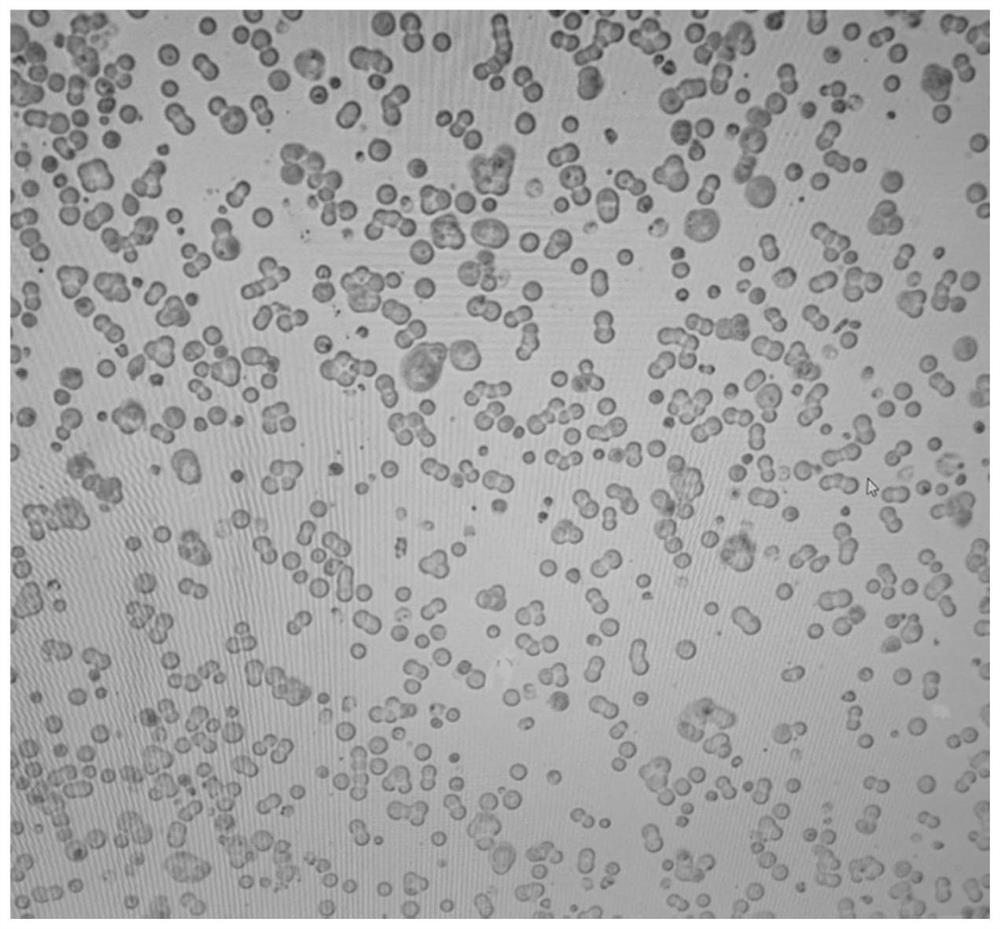
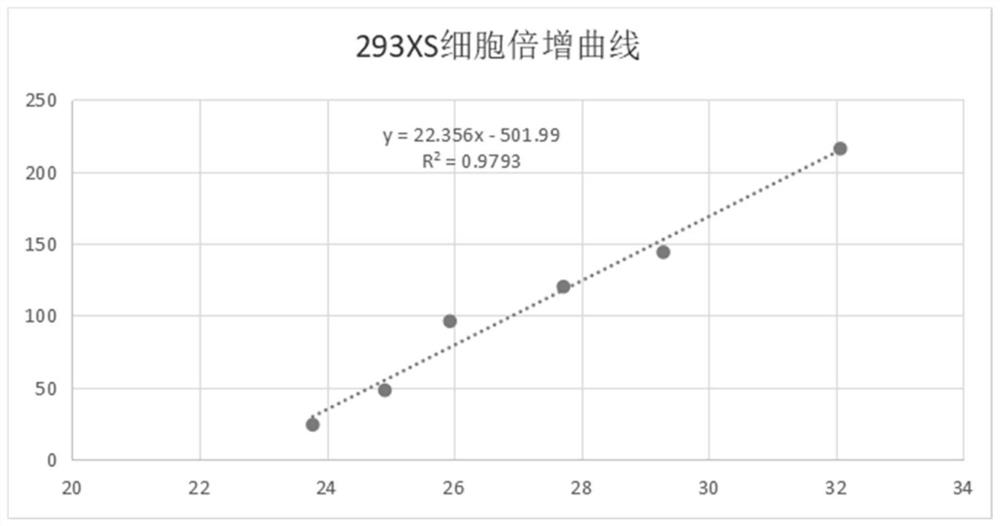
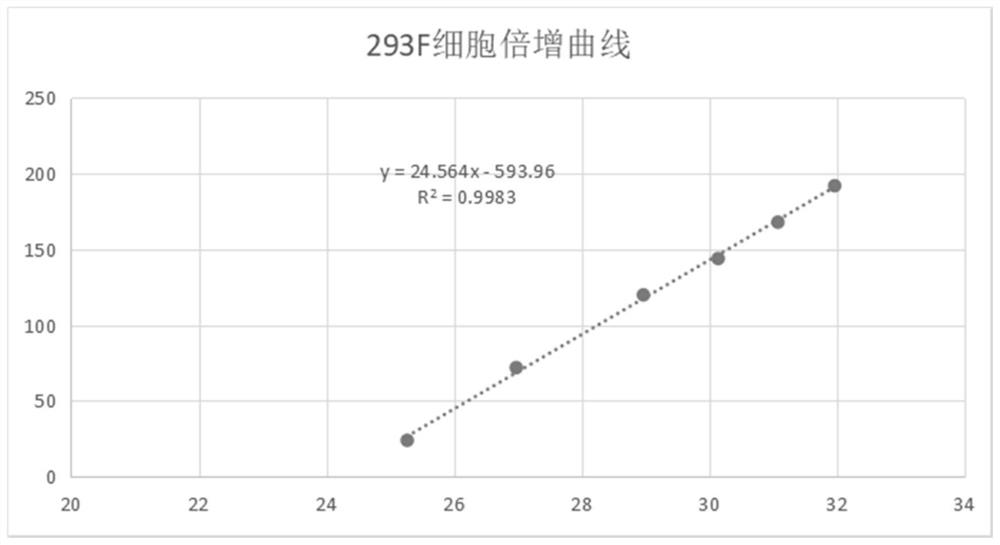
Preparation method for serum-free and suspension culture of HEK-293 cells and application of serum-free and suspension culture of HEK-293 cells
A technology of HEK-293 and serum-free medium, which is applied in the field of preparation of HEK-293 cell serum-free and suspension culture, which can solve the problems of contamination of exogenous microorganisms and pathogenic factors, difficulty in large-scale production, and difficult quality control , to achieve high transfection efficiency and toxin production capacity, stable cell growth state, and high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Preparation of serum-free and suspension culture of HEK-293 cells
[0072] 1. Preparation method:
[0073] (1). Serum-free acclimatization:
[0074] a. Recovery of HEK-293 cells
[0075] Take a frozen HEK-293 cell, put it into a 37°C water bath to thaw, shake
[0076] Let it melt as soon as possible in 45ml DMEM solution containing 10% FBS; mix well, centrifuge at 300g for 5min, discard the supernatant; according to 1.0~1.1×10 7 / dish, the resulting pellet was resuspended with 10ml DMEM medium containing 10% FBS, mixed evenly and cultured;
[0077] b. Culture HEK-293 cells in DMEM medium with decreasing concentration gradient of fetal bovine serum
[0078] When the cell confluency is about 80%, the supernatant is discarded, digested with Tryple and passaged to the cell culture dish. At this time, the medium was replaced with DMEM medium containing 5% FBS, cultured at 37° C. in a 5% CO2 incubator, and the growth status of the cells was observed. Fresh medi...
Embodiment 2
[0102] Example 2: Cellular virus packaging and toxin production capacity
[0103] 1. Method steps:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com