Lentivirus recombinant expression vector/recombinant lentivirus, as well as application, host cell and preparing method thereof
A technology of recombinant lentivirus and expression vector, which can be used in viruses/phages, botanical equipment and methods, biochemical equipment and methods, etc., and can solve the problems of difficult transfection, difficult to achieve high-efficiency expression of target genes, and lack of stable cell lines. , to achieve the effect of a wide range of applications, a wide host range, and high transcription efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
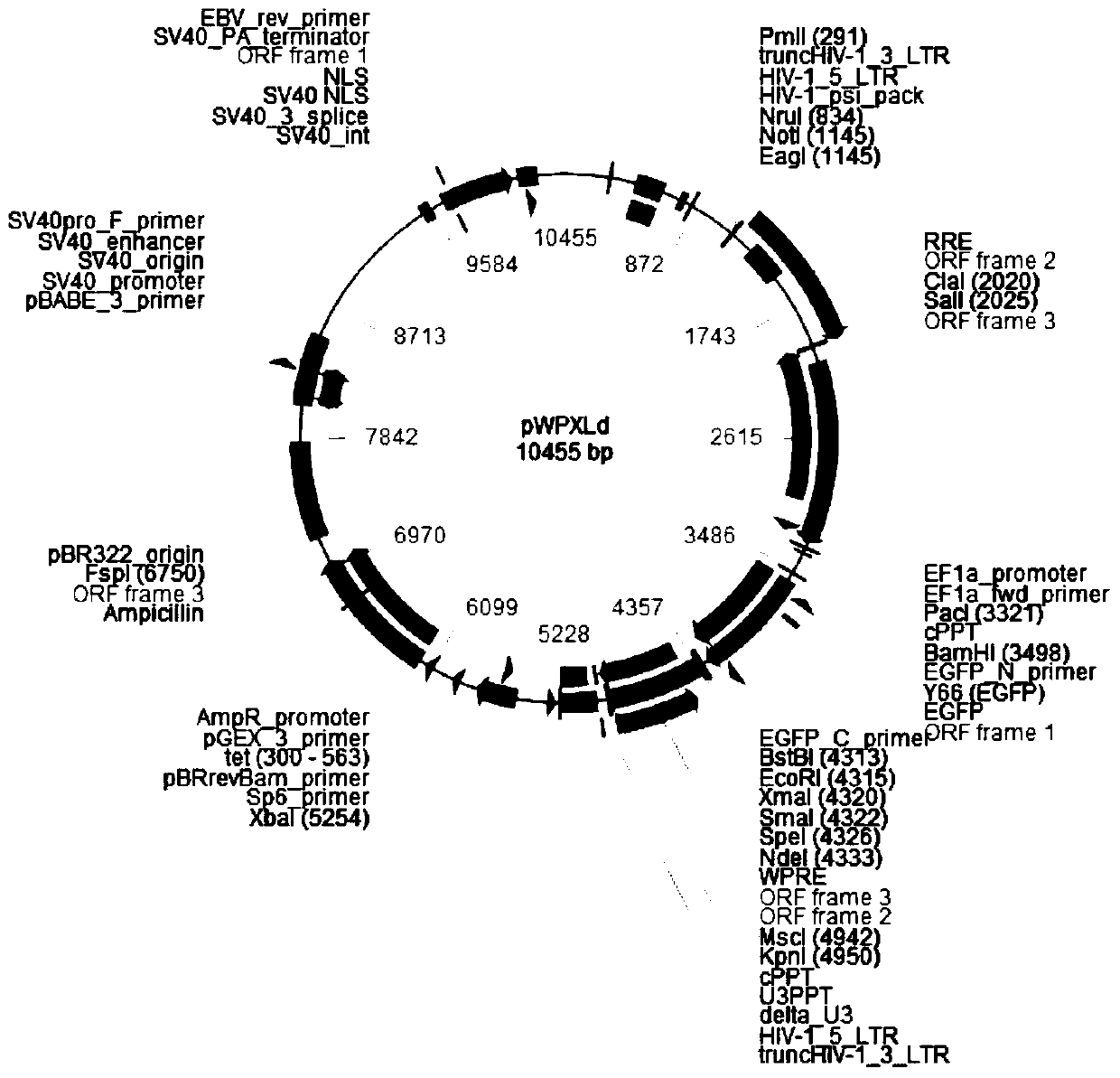
[0068] A method for constructing a human-derived estrogen-related receptor alpha overexpression lentiviral recombinant expression vector (pWPXLd-hERRα), comprising the following steps:
[0069] 1. Amplification and purification of hERRα target gene
[0070] 1.1 Primer design and synthesis: use primerpremier5.0 software to design primers as follows:
[0071] F (SEQ ID NO: 2): 5'-GGGTTTAAACATGTCCAGCCAGGTG-3';
[0072] R (SEQ ID NO: 3): 5'-CCCCCGGGTCAGTCCATCATGGCC-3'.
[0073] F and R introduce restriction endonucleases PmeI and SmaI cutting sites, respectively.
[0074] 1.2 Use the pCMX-hERRα plasmid as a template to amplify the target fragment (please modify the yellow highlighted template) Use the 500-fold diluted pCMX-hERRα plasmid as a template, and use the above primers for PCR amplification. The reaction conditions for amplification are: 94°C Pre-denatured for 2 minutes, denatured at 98°C for 10 seconds, annealed at 59°C for 30 seconds, extended at 68°C for 1 minute and...
Embodiment 2
[0164] A method for expressing an estrogen-related receptor alpha overexpression lentiviral recombinant expression vector (pWPXLd-hERRα) in 293T and HepG2 cell lines, comprising the following steps:
[0165] 3.1 Lentivirus packaging, concentration and infection
[0166] 1) 293FT cells were plated one day in advance (use DMEM complete medium with ordinary pH 7.4 when plated), and the 293FT cells normally cultured in a 6cm plate with a density of about 85-95% were divided into two new 6cm plates ( About 6*105 cells), or directly plate all in a 10cm plate; 12-18h after plating is the best transfection time, if it exceeds 24h, do not transfect, it is recommended to re-plate;
[0167] 2) Transfection: Before transfection, replace the liquid with DMEM complete medium at pH 7.4 (add 3ml of medium to a 6cm plate)
[0168] Take two 1.5ml Dorf tubes, add 300ul DMEM complete medium respectively, dissolve the plasmid pWPXLd–hERRα4ug; psPAX23ug; After uniformity, transfer it into the dor...
Embodiment 3
[0188] This example 3 provides a method for determining the virus titer of the 293T cells transfected with pWPXLd-hERRα provided in Example 2, including the following steps:
[0189] a. Dilute the well-growing 293T cells to 1×10 after digestion and counting 5 / ml, add to 96-well plate, 100ul / well, prepare 10 wells for each virus, place at 37℃, 5%CO 2 Incubate overnight in an incubator.
[0190] b. After 24 hours, the virus was serially diluted 10 times, and 12 serial dilutions were performed.
[0191] c. Discard the original culture medium of the 96-well plate, add the diluted virus solution, and set a blank control. Polybrene (polybrene, working concentration is 6ug / ml) was added to the culture medium to promote virus infection of cells.
[0192] d. Add 100ul culture solution to each well after 48h.
[0193] e. On the fifth day of culture, observe the results under a fluorescent microscope, and count the number of fluorescent cell clones in the two lowest dilution wells w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com