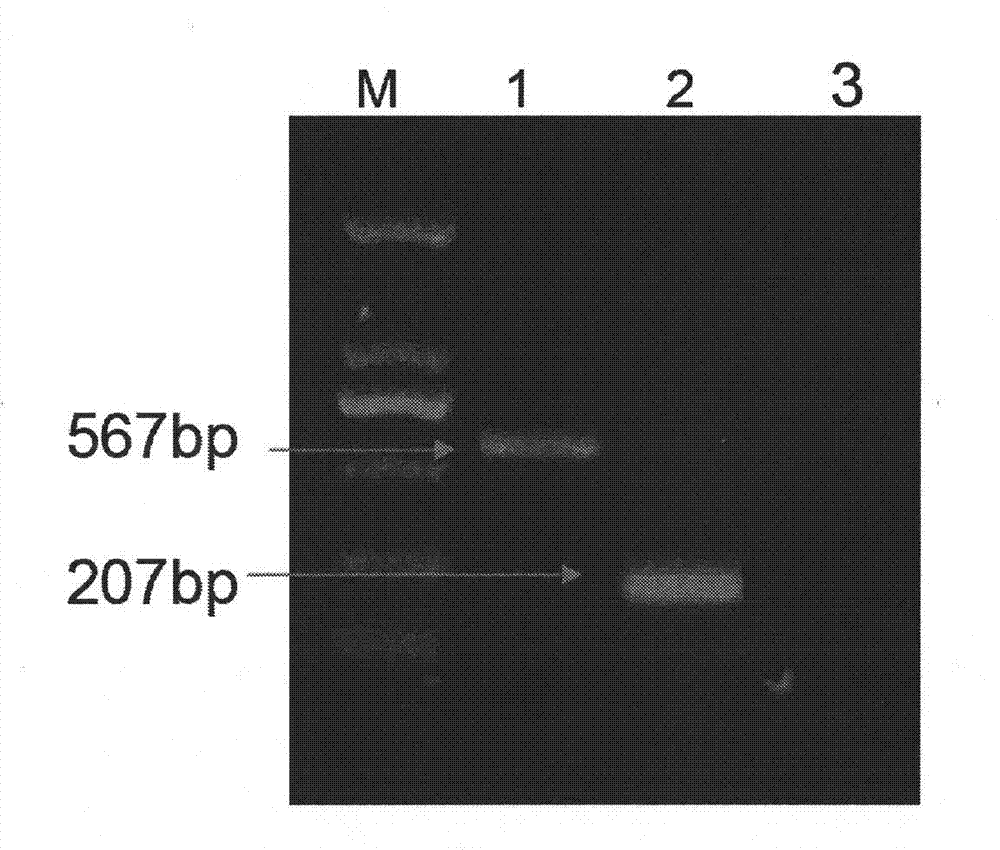
Combined live vaccine against porcine reproductive and respiratory syndrome and pseudorabies, and preparation method thereof
A technology for respiratory syndrome and porcine pseudorabies, applied in antiviral agents, pharmaceutical formulations, viral antigen components, etc., can solve problems such as difficult to achieve effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] Compared with the prior art, the preparation method provided by the invention has many advantages. For example, its preparation process is simple and stable, easy to operate, high virus content, small batch-to-batch variation, easy quality control, can significantly improve vaccine yield and quality, and reduce allergic reactions, etc. The porcine reproductive and respiratory syndrome and pseudorabies dual live vaccine obtained by the preparation method of the present invention has good safety and high immune efficacy, and has better immune protection against the virulent attack of porcine reproductive and respiratory syndrome and pseudorabies.
[0081] The invention also provides the PRRSV and PRV dual vaccine composition prepared by the method of the invention.
[0082] The invention also provides the use of the vaccine composition in the preparation of biological products for preventing or treating porcine reproductive and respiratory syndrome and porcine pseudorabie...
Embodiment 1
[0091] The preparation of embodiment 1 highly pathogenic porcine reproductive and respiratory syndrome, pseudorabies dual live vaccine
[0092] Prepare highly pathogenic porcine reproductive and respiratory syndrome and pseudorabies dual live vaccine by the following method:
[0093] (1) Select susceptible cells as cells for seedling preparation: African green monkey kidney cells (Marc-145) were selected as susceptible cells of PRRSV, and Marc-145, bovine kidney cells (MDBK) and bovine testicular passage cells (BT) were selected. Passage cells are susceptible to PRV.
[0094] (2) Passage and culture of cells for seedling production: the above-mentioned passage cells are digested and passaged by EDTA-trypsin cell dispersion liquid, and continue to be cultured with cell growth medium. The temperature is 36-37°C.
[0095] (3) Propagation of poisonous species for production:
[0096] A. Propagation of virus seeds for production of highly pathogenic PRRSV live vaccine: In the ME...
Embodiment 2
[0106] The purity test of embodiment 2 highly pathogenic PRRSV, PRV dual live vaccine
[0107] According to the description on pages 15, 19, and 20 of the appendix of "The Veterinary Pharmacopoeia of the Republic of China" (2005 edition), the double live vaccine prepared in Example 1 is tested, and the result shows that the double live vaccine has no bacteria, mold and mycoplasma Growth without exogenous virus contamination.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com