Combined inactivated vaccine of Newcastle disease and H9 subtype avian influenza and preparation method thereof
An inactivated vaccine, chicken Newcastle disease technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, virus antigen components, etc., can solve the problems of difficult vaccination, large immune dose, large immune stress, etc. Long-term, small immune dose, and the effect of reducing stress response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
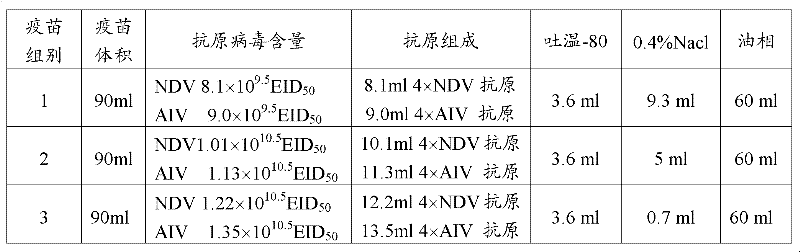
[0019] The preparation of embodiment 1 chicken Newcastle disease and H9 subtype avian influenza double concentrated inactivated vaccine
[0020] 1 Preparation of poisonous seeds for production
[0021] 1. Propagation and identification of virus species of 1H9HL strain
[0022] 1.1.1 Breeding of virus seeds Avian influenza virus A / Chicken / Henan / Luoyang / HL / 2001 (H9N2) (abbreviated as HL strain). The HL strain freeze-dried virus was properly diluted with sterilized physiological saline (10 -3 ~10 -4 ), 0.1ml per allantoic cavity was inoculated with 10-day-old SPF embryos, and incubated at 37°C for 96 hours. During this period, the dead embryos before 48 hours were discarded. After 48 hours, the embryos were photographed once every 4 to 6 hours, and the dead embryos were removed in time. After 96 hours, take them all out, put them in the refrigerator at 2-8°C for more than 12 hours, harvest the chicken embryo fluids separately, put them into sterilized containers, mix the chick...
Embodiment 2
[0060] Embodiment 2, the effect measurement of chicken Newcastle disease and H9 subtype avian influenza dual concentrated inactivated vaccine
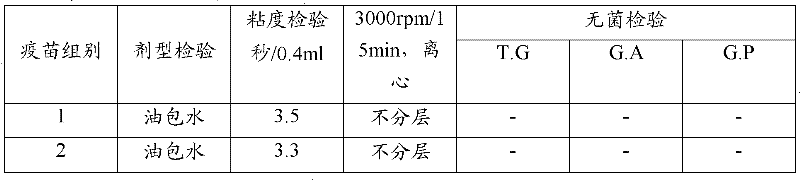
[0061] 1 physical properties
[0062] Appearance The finished preparation is a white homogeneous emulsion when observed with the naked eye.
[0063] The formulation is water-in-oil. Take a clean straw, suck a small amount of vaccine and drop it on the surface of cold water, in the shape of oil droplets, without spreading.
[0064] Stability Put the vaccine in a 10ml centrifuge tube, centrifuge at 3000rpm for 15min, and the water phase ≤0.5ml will precipitate out at the bottom of the tube.
[0065] Viscosity Use a 1ml straw with an outlet diameter of 1.2mm to draw 1.0ml of vaccine at about 25°C, let it flow out vertically and naturally, record the time required for the outflow of 0.4ml, within 8s, the inspection is qualified.
[0066] 2 The sterility test was carried out according to the P448 method of "Regulations of Veterinary Biol...
Embodiment 3
[0081] Embodiment 3 Newcastle disease and H9 subtype avian influenza dual concentrated inactivated vaccine and the immune effect comparative test of chicken Newcastle disease (LaSota strain) and H9 subtype avian influenza (HL strain) dual inactivated vaccine
[0082]1. Whole body and local immune response of small day-old chickens Get 20 SPF chickens of 1-10 days age, divide into two groups, one group is immunized with the prepared vaccine of vaccine preparation formula 1 in Example 1, and each intramuscular injection 0.1ml Another group was immunized with the vaccine purchased from the market (Newcastle disease, avian influenza H9 subtype dual inactivated vaccine; Qilu Animal Health Products Company, batch number 20110230), and each muscle was injected with 0.3ml of the dual vaccine, continuously for 14 days after the injection Observe systemic reactions and local reactions, see Table 4 for details.
[0083] Table 4 Immunization observation results of young day-old chickens ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com