Porcine epidemic diarrhea virus variant inactivated vaccine and application thereof
A porcine epidemic diarrhea and inactivated vaccine technology, applied in the direction of viruses, antiviral agents, virus antigen components, etc., can solve the problems of lack of immunogenicity and achieve good immunogenicity, high antibody titer, and resistance to attack
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: Screening of porcine epidemic diarrhea virus PEDV / CH / BJ / 2014 strain
[0013] 1 Isolation and identification of porcine epidemic diarrhea virus PEDV / CH / BJ / 2014 strain
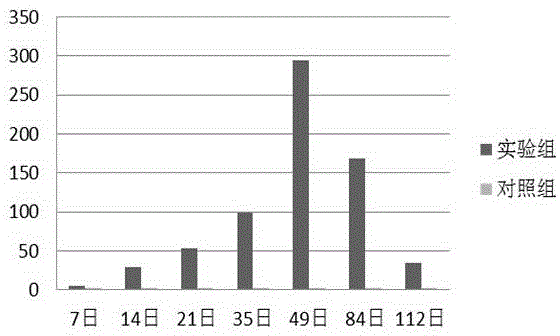
[0014] During the epidemiological investigation, a strain of porcine epidemic diarrhea virus (named PEDV / CH / BJ / 2014 strain) was isolated from the small intestine contents of piglets with diarrhea submitted to a pig farm in Beijing. Porcine epidemic diarrhea virus RT- The PCR test was positive, and the 2nd generation of blind passage had cytopathic changes, and regular and stable cytopathic changes appeared in the 3rd generation, and the virus content could reach 5×10 in the 5th generation. 5 TCID 50 / mL, which can be neutralized by anti-porcine epidemic diarrhea virus-specific serum, the porcine epidemic diarrhea virus PEDV / CH / BJ / 2014 strain was preserved at No. 1 Beichen West Road, Chaoyang District, Beijing on December 4, 2014 No. 3 General Microbiology Center of Chinese Academy of Scien...
Embodiment 2
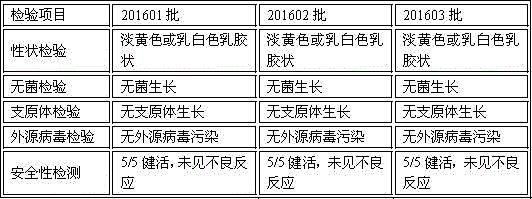
[0028] Example 2 Safety Test of Porcine Epidemic Diarrhea Inactivated Vaccine
[0029] 1 Experimental method
[0030] Overdose test on pregnant sows 12 pregnant sows that were negative for porcine epidemic diarrhea neutralizing antibodies and antigens 4-6 weeks before delivery were randomly divided into 3 groups. Groups 1, 2 and 3 were injected intramuscularly with 201601 , 201602, 201603 batches of porcine epidemic diarrhea inactivated vaccines each had 2 parts per head, and the fourth group was not injected as the control group, and the sows were observed until the sows gave birth.
[0031] 2 results
[0032] Results of the overdose test Compared with the control group, the 9 pregnant sows in the 3 batches of vaccine immunization groups had no abnormalities in food intake, drinking water, pregnancy and litter, and no adverse reactions were found at the injection site.
Embodiment 3
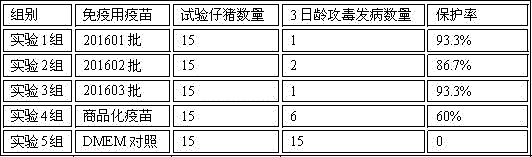
[0033] Example 3 Efficacy Test of Porcine Epidemic Diarrhea Inactivated Vaccine
[0034] 1 Experimental method
[0035] Fifteen pregnant sows at the same pre-delivery date who were negative for neutralizing antibodies and antigens in porcine epidemic diarrhea 5 weeks before delivery were randomly divided into 5 groups with 3 heads in each group. Group 1 intramuscular injection of 201601 batches of porcine epidemic diarrhea virus inactivated vaccine 1 head / head; group 2 intramuscular injection of 201602 batches of porcine epidemic diarrhea virus inactivated vaccine 1 head / head; group 3 intramuscular injection of 201603 batches of pigs Epidemic diarrhea virus inactivated vaccine 1 head / head; Group 4 intramuscular injection of commercial porcine transmissible gastroenteritis-porcine epidemic diarrhea dual inactivated vaccine 1 head / head; Group 5 muscle sterile cell culture Liquid DMEM 4 mL / head served as the control group.
[0036] After the sows gave birth, 15 3-day-old piglet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com